Reprogramming for cardiac regeneration
- PMID: 25763379
- PMCID: PMC4352683
- DOI: 10.5339/gcsp.2014.44
Reprogramming for cardiac regeneration
Abstract
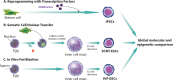
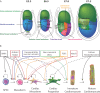
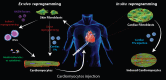
Treatment of cardiovascular diseases remains challenging considering the limited regeneration capacity of the heart muscle. Developments of reprogramming strategies to create in vitro and in vivo cardiomyocytes have been the focus point of a considerable amount of research in the past decades. The choice of cells to employ, the state-of-the-art methods for different reprogramming strategies, and their promises and future challenges before clinical entry, are all discussed here.
Figures




References
-
- Lancaster MA, Knoblich JA. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science. 2014;345(6194):1247125. - PubMed
-
- Michael Fischberg JBG, Tom R. Elsdale nuclear transplantation in Xenopus laevis. Nature. 1958;(181):424.
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
