Developmental programming: exposure to testosterone excess disrupts steroidal and metabolic environment in pregnant sheep
- PMID: 25763641
- PMCID: PMC4430607
- DOI: 10.1210/en.2014-2006
Developmental programming: exposure to testosterone excess disrupts steroidal and metabolic environment in pregnant sheep
Abstract
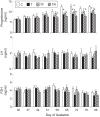
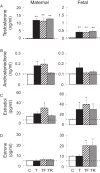
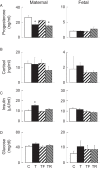
Gestational exposure to excess T leads to intrauterine growth restriction, low birth weight, and adult metabolic/reproductive disorders in female sheep. We hypothesized that as early mediators of such disruptions, gestational T disrupts steroidal and metabolic homeostasis in both the mother and fetus by both androgenic and metabolic pathways. Maternal blood samples were measured weekly for levels of insulin, glucose, and progesterone from four groups of animals: control; gestational T (twice weekly im injections of 100 mg of T propionate from d 30 to d 90 of gestation); T plus an androgen antagonist, flutamide (15 mg/kg·d oral; T-Flutamide); and T plus the insulin sensitizer, rosiglitazone (0.11 mg/kg·d oral; T-Rosi) (n = 10-12/group). On day 90 of gestation, maternal and umbilical cord samples were collected after a 48-hour fast from a subset (n = 6/group) for the measurement of steroids, free fatty acids, amino acids, and acylcarnitines. Gestational T decreased maternal progesterone levels by 36.5% (P < .05), which was prevented by flutamide showing direct androgenic mediation. Gestational T also augmented maternal insulin levels and decreased medium chained acylcarnitines, suggesting increased mitochondrial fatty acid oxidation. These changes were prevented by rosiglitazone, suggesting alterations in maternal fuel use. Gestational T-induced increases in fetal estradiol were not prevented by either cotreatment. Gestational T disrupted associations of steroids with metabolites and progesterone with acylcarnitines, which was prevented either by androgen antagonist or insulin sensitizer cotreatment. These findings suggest a future combination of these treatments might be required to prevent alteration in maternal/fetal steroidal and metabolic milieu(s).
Figures
References
-
- Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;(suppl 6):588S–595S. - PubMed
-
- Fowden AL, Giussani DA, Forhead AJ. Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda). 2006;21:29–37. - PubMed
-
- Aceti A, Santhakumaran S, Logan KM, et al. The diabetic pregnancy and offspring blood pressure in childhood: a systematic review and meta-analysis. Diabetologia. 2012;11:3114–3127. - PubMed
-
- Poston L. Developmental programming and diabetes—the human experience and insight from animal models. Best Pract Res Clin Endocrinol Metab. 2010;4:541–552. - PubMed
-
- Fowden AL, Forhead AJ. Endocrine regulation of feto-placental growth. Horm Res. 2009;72:257–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

