TRIM26 negatively regulates interferon-β production and antiviral response through polyubiquitination and degradation of nuclear IRF3
- PMID: 25763818
- PMCID: PMC4357427
- DOI: 10.1371/journal.ppat.1004726
TRIM26 negatively regulates interferon-β production and antiviral response through polyubiquitination and degradation of nuclear IRF3
Abstract
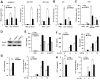
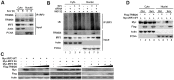
Virus infection leads to the activation of transcription factor IRF3 and subsequent production of type I inteferons, which induce the transcription of various antiviral genes called interferon stimulated genes (ISGs) to eliminate viral infection. IRF3 activation requires phosphorylation, dimerization and nuclear translocation. However, the mechanisms for the termination of IRF3 activation in nucleus are elusive. Here we report the identification of TRIM26 to negatively regulate IFN-β production and antiviral response by targeting nuclear IRF3. TRIM26 bound to IRF3 and promoted its K48-linked polyubiquitination and degradation in nucleus. TRIM26 degraded WT IRF3 and the constitutive active mutant IRF3 5D, but not the phosphorylation deficient mutant IRF3 5A. Furthermore, IRF3 mutant in the Nuclear Localization Signal (NLS), which could not move into nucleus, was not degraded by TRIM26. Importantly, virus infection promoted TRIM26 nuclear translocation, which was required for IRF3 degradation. As a consequence, TRIM26 attenuated IFN-β promoter activation and IFN-β production downstream of TLR3/4, RLR and DNA sensing pathways. TRIM26 transgenic mice showed much less IRF3 activation and IFN-β production, while increased virus replication. Our findings delineate a novel mechanism for the termination of IRF3 activation in nucleus through TRIM26-mediated IRF3 ubiquitination and degradation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Janeway CA Jr, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20: 197–216. - PubMed
-
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, et al. (2004) The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 5: 730–737. - PubMed
-
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, et al. (2006) Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441: 101–105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

