SOCS3 silencing attenuates eosinophil functions in asthma patients
- PMID: 25764157
- PMCID: PMC4394485
- DOI: 10.3390/ijms16035434
SOCS3 silencing attenuates eosinophil functions in asthma patients
Abstract
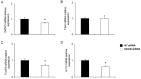
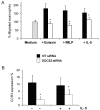
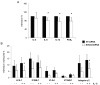
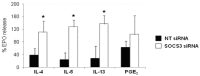
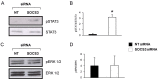
Eosinophils are one of the key inflammatory cells in asthma. Eosinophils can exert a wide variety of actions through expression and secretion of multiple molecules. Previously, we have demonstrated that eosinophils purified from peripheral blood from asthma patients express high levels of suppressor of cytokine signaling 3 (SOCS3). In this article, SOCS3 gene silencing in eosinophils from asthmatics has been carried out to achieve a better understanding of the suppressor function in eosinophils. SOCS3 siRNA treatment drastically reduced SOCS3 expression in eosinophils, leading to an inhibition of the regulatory transcription factors GATA-3 and FoxP3, also interleukin (IL)-10; in turn, an increased STAT3 phosphorilation was observed. Moreover, SOCS3 abrogation in eosinophils produced impaired migration, adhesion and degranulation. Therefore, SOCS3 might be regarded as an important regulator implicated in eosinophil mobilization from the bone marrow to the lungs during the asthmatic process.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

