Beyond iron: non-classical biological functions of bacterial siderophores
- PMID: 25764171
- PMCID: PMC4375017
- DOI: 10.1039/c4dt03559c
Beyond iron: non-classical biological functions of bacterial siderophores
Abstract
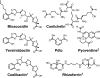
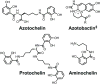
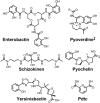
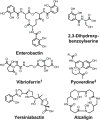
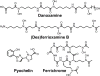
Bacteria secrete small molecules known as siderophores to acquire iron from their surroundings. For over 60 years, investigations into the bioinorganic chemistry of these molecules, including fundamental coordination chemistry studies, have provided insight into the crucial role that siderophores play in bacterial iron homeostasis. The importance of understanding the fundamental chemistry underlying bacterial life has been highlighted evermore in recent years because of the emergence of antibiotic-resistant bacteria and the need to prevent the global rise of these superbugs. Increasing reports of siderophores functioning in capacities other than iron transport have appeared recently, but reports of "non-classical" siderophore functions have long paralleled those of iron transport. One particular non-classical function of these iron chelators, namely antibiotic activity, was documented before the role of siderophores in iron transport was established. In this Perspective, we present an exposition of past and current work into non-classical functions of siderophores and highlight the directions in which we anticipate that this research is headed. Examples include the ability of siderophores to function as zincophores, chalkophores, and metallophores for a variety of other metals, sequester heavy metal toxins, transport boron, act as signalling molecules, regulate oxidative stress, and provide antibacterial activity.
Figures


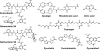
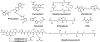


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

