Magnetoencephalography in the study of brain dynamics
- PMID: 25764254
- PMCID: PMC4370437
Magnetoencephalography in the study of brain dynamics
Abstract
To progress toward understanding of the mechanisms underlying the functional organization of the human brain, either a bottom-up or a top-down approach may be adopted. The former starts from the study of the detailed functioning of a small number of neuronal assemblies, while the latter tries to decode brain functioning by considering the brain as a whole. This review discusses the top-down approach and the use of magnetoencephalography (MEG) to describe global brain properties. The main idea behind this approach is that the concurrence of several areas is required for the brain to instantiate a specific behavior/functioning. A central issue is therefore the study of brain functional connectivity and the concept of brain networks as ensembles of distant brain areas that preferentially exchange information. Importantly, the human brain is a dynamic device, and MEG is ideally suited to investigate phenomena on behaviorally relevant timescales, also offering the possibility of capturing behaviorally-related brain connectivity dynamics.
Figures
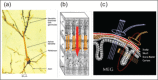

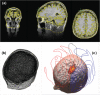
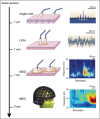
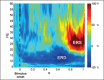
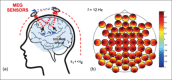
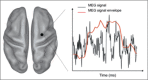
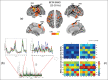
References
-
- Ahlfors SP, Simpson GV, Dale AM, et al. Spatiotemporal activity of a cortical network for processing visual motion revealed by MEG and fMRI. J Neurophysiol. 1999;82:2545–2555. - PubMed
-
- Baillet S, Mosher JC, Leahy RM. Electromagnetic brain mapping. IEEE Signal Processing Magazine. 2001;18:14–30.
-
- Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
