The Old and New Testaments of gene regulation. Evolution of multi-subunit RNA polymerases and co-evolution of eukaryote complexity with the RNAP II CTD
- PMID: 25764332
- PMCID: PMC4215175
- DOI: 10.4161/trns.28674
The Old and New Testaments of gene regulation. Evolution of multi-subunit RNA polymerases and co-evolution of eukaryote complexity with the RNAP II CTD
Abstract
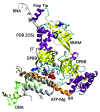
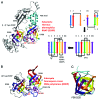
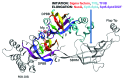
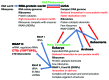
I relate a story of genesis told from the point of view of multi-subunit RNA polymerases (RNAPs) including an Old Testament (core RNAP motifs in all cellular life) and a New Testament (the RNAP II heptad repeat carboxy terminal domain (CTD) and CTD interactome in eukarya). The Old Testament: at their active site, one class of eukaryotic interfering RNAP and ubiquitous multi-subunit RNAPs each have two-double psi β barrel (DPBB) motifs (a distinct pattern for compact 6-β sheet barrels). Between β sheets 2 and 3 of the β subunit type DPBB of all multi-subunit RNAPs is a sandwich barrel hybrid motif (SBHM) that interacts with conserved initiation and elongation factors required to utilize a DNA template. Analysis of RNAP core protein motifs, therefore, indicates that RNAP evolution can be traced from the RNA-protein world to LUCA (the last universal common ancestor) branching to LECA (the last eukaryotic common ancestor) and to the present day, spanning about 4 billion years. The New Testament: in the eukaryotic lineage, I posit that splitting RNAP functions into RNAPs I, II and III and innovations developed around the CTD heptad repeat of RNAP II and the extensive CTD interactome helps to describe how greater structural, cell cycle, epigenetic and signaling complexity co-evolved in eukaryotes relative to eubacteria and archaea.
Keywords: Chromatin and transcription; Eukaryotic transcription; Prokaryotic transcription; RNA polymerases; transcriptional elongation; transcriptional initiation; transcriptional termination.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
