ATPase-dependent control of the Mms21 SUMO ligase during DNA repair
- PMID: 25764370
- PMCID: PMC4357442
- DOI: 10.1371/journal.pbio.1002089
ATPase-dependent control of the Mms21 SUMO ligase during DNA repair
Abstract
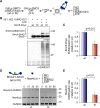
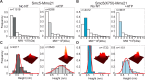
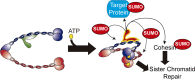
Modification of proteins by SUMO is essential for the maintenance of genome integrity. During DNA replication, the Mms21-branch of the SUMO pathway counteracts recombination intermediates at damaged replication forks, thus facilitating sister chromatid disjunction. The Mms21 SUMO ligase docks to the arm region of the Smc5 protein in the Smc5/6 complex; together, they cooperate during recombinational DNA repair. Yet how the activity of the SUMO ligase is controlled remains unknown. Here we show that the SUMO ligase and the chromosome disjunction functions of Mms21 depend on its docking to an intact and active Smc5/6 complex, indicating that the Smc5/6-Mms21 complex operates as a large SUMO ligase in vivo. In spite of the physical distance separating the E3 and the nucleotide-binding domains in Smc5/6, Mms21-dependent sumoylation requires binding of ATP to Smc5, a step that is part of the ligase mechanism that assists Ubc9 function. The communication is enabled by the presence of a conserved disruption in the coiled coil domain of Smc5, pointing to potential conformational changes for SUMO ligase activation. In accordance, scanning force microscopy of the Smc5-Mms21 heterodimer shows that the molecule is physically remodeled in an ATP-dependent manner. Our results demonstrate that the ATP-binding activity of the Smc5/6 complex is coordinated with its SUMO ligase, through the coiled coil domain of Smc5 and the physical remodeling of the molecule, to promote sumoylation and chromosome disjunction during DNA repair.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

