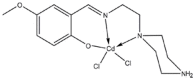
Apoptotic effect of novel Schiff based CdCl₂(C₁₄H₂₁N₃O₂) complex is mediated via activation of the mitochondrial pathway in colon cancer cells
- PMID: 25764970
- PMCID: PMC4649862
- DOI: 10.1038/srep09097
Apoptotic effect of novel Schiff based CdCl₂(C₁₄H₂₁N₃O₂) complex is mediated via activation of the mitochondrial pathway in colon cancer cells
Erratum in
-
Corrigendum: Apoptotic effect of novel Schiff Based CdCl2(C14H21N3O2) complex is mediated via activation of the mitochondrial pathway in colon cancer cells.Sci Rep. 2017 Jun 5;7:46793. doi: 10.1038/srep46793. Sci Rep. 2017. PMID: 28582385 Free PMC article.
Abstract
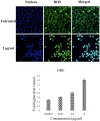
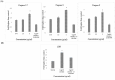
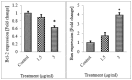
The development of metal-based agents has had a tremendous role in the present progress in cancer chemotherapy. One well-known example of metal-based agents is Schiff based metal complexes, which hold great promise for cancer therapy. Based on the potential of Schiff based complexes for the induction of apoptosis, this study aimed to examine the cytotoxic and apoptotic activity of a CdCl2(C14H21N3O2) complex on HT-29 cells. The complex exerted a potent suppressive effect on HT-29 cells with an IC50 value of 2.57 ± 0.39 after 72 h of treatment. The collapse of the mitochondrial membrane potential and the elevated release of cytochrome c from the mitochondria to the cytosol indicate the involvement of the intrinsic pathway in the induction of apoptosis. The role of the mitochondria-dependent apoptotic pathway was further proved by the significant activation of the initiator caspase-9 and the executioner caspases-3 and -7. In addition, the activation of caspase-8, which is associated with the suppression of NF-κB translocation to the nucleus, also revealed the involvement of the extrinsic pathway in the induced apoptosis. The results suggest that the CdCl2(C14H21N3O2) complex is able to induce the apoptosis of colon cancer cells and is a potential candidate for future cancer studies.
Figures








References
-
- Siegel R., DeSantis C., Virgo K., Stein K. & Mariotto T. Cancer treatment and survivorship statistics. CA Cancer J Clin. 62, 220–41 (2012). - PubMed
-
- Pan M. H., Chiou Y. S., Wang Y. J., Ho C. T. & Lin J. K. Multistage carcinogenesis process as molecular targets in cancer chemoprevention by epicatechin-3-gallate. Food Funct, 2, 101–10 (2011). - PubMed
-
- Fearon E. Molecular genetics of colorectal cancer. Annu Rev Pathol-Mech. 6, 479–507 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

