Predicting functional and regulatory divergence of a drug resistance transporter gene in the human malaria parasite
- PMID: 25765049
- PMCID: PMC4352545
- DOI: 10.1186/s12864-015-1261-6
Predicting functional and regulatory divergence of a drug resistance transporter gene in the human malaria parasite
Abstract
Background: The paradigm of resistance evolution to chemotherapeutic agents is that a key coding mutation in a specific gene drives resistance to a particular drug. In the case of resistance to the anti-malarial drug chloroquine (CQ), a specific mutation in the transporter pfcrt is associated with resistance. Here, we apply a series of analytical steps to gene expression data from our lab and leverage 3 independent datasets to identify pfcrt-interacting genes. Resulting networks provide insights into pfcrt's biological functions and regulation, as well as the divergent phenotypic effects of its allelic variants in different genetic backgrounds.
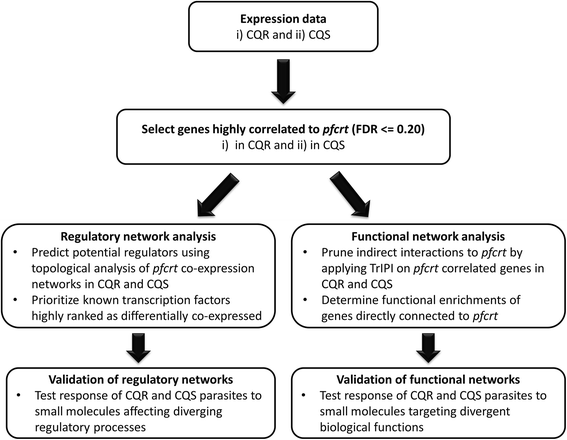
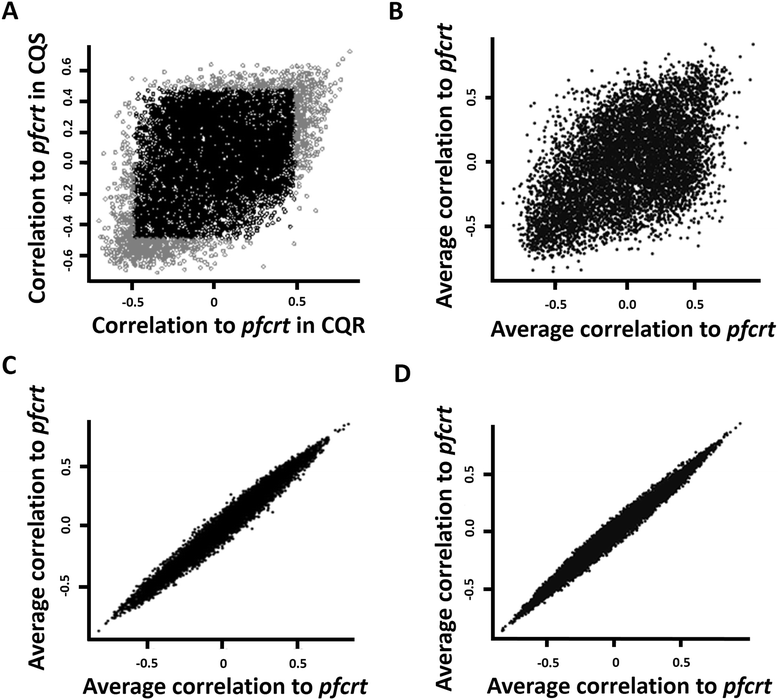
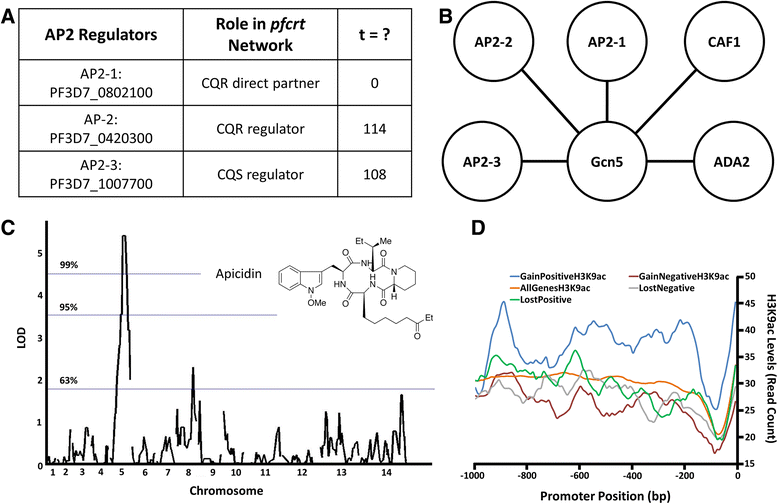
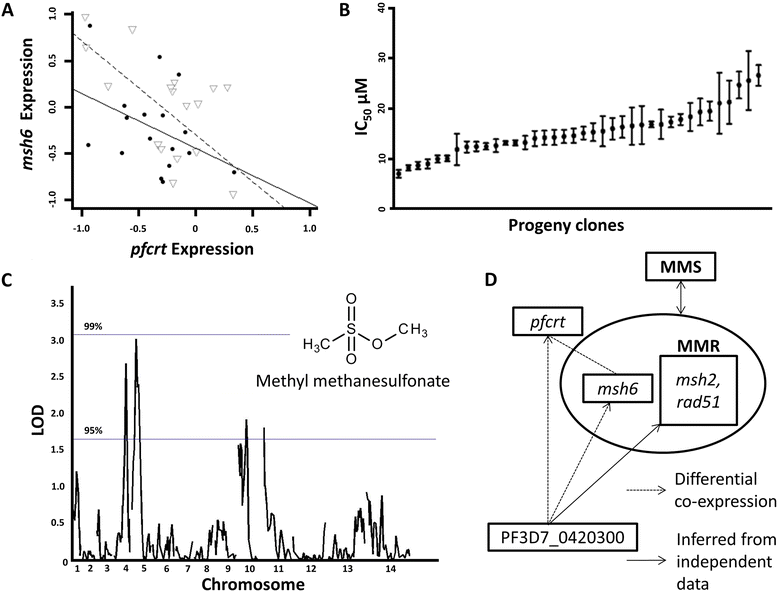
Results: To identify pfcrt-interacting genes, we analyze pfcrt co-expression networks in 2 phenotypic states - CQ-resistant (CQR) and CQ-sensitive (CQS) recombinant progeny clones - using a computational approach that prioritizes gene interactions into functional and regulatory relationships. For both phenotypic states, pfcrt co-expressed gene sets are associated with hemoglobin metabolism, consistent with CQ's expected mode of action. To predict the drivers of co-expression divergence, we integrate topological relationships in the co-expression networks with available high confidence protein-protein interaction data. This analysis identifies 3 transcriptional regulators from the ApiAP2 family and histone acetylation as potential mediators of these divergences. We validate the predicted divergences in DNA mismatch repair and histone acetylation by measuring the effects of small molecule inhibitors in recombinant progeny clones combined with quantitative trait locus (QTL) mapping.
Conclusions: This work demonstrates the utility of differential co-expression viewed in a network framework to uncover functional and regulatory divergence in phenotypically distinct parasites. pfcrt-associated co-expression in the CQ resistant progeny highlights CQR-specific gene relationships and possible targeted intervention strategies. The approaches outlined here can be readily generalized to other parasite populations and drug resistances.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

