Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury
- PMID: 25765066
- PMCID: PMC4445125
- DOI: 10.1126/science.aaa2958
Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury
Abstract
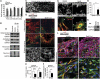
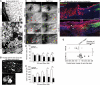
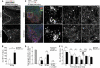
After central nervous system (CNS) injury, inhibitory factors in the lesion scar and poor axon growth potential prevent axon regeneration. Microtubule stabilization reduces scarring and promotes axon growth. However, the cellular mechanisms of this dual effect remain unclear. Here, delayed systemic administration of a blood-brain barrier-permeable microtubule-stabilizing drug, epothilone B (epoB), decreased scarring after rodent spinal cord injury (SCI) by abrogating polarization and directed migration of scar-forming fibroblasts. Conversely, epothilone B reactivated neuronal polarization by inducing concerted microtubule polymerization into the axon tip, which propelled axon growth through an inhibitory environment. Together, these drug-elicited effects promoted axon regeneration and improved motor function after SCI. With recent clinical approval, epothilones hold promise for clinical use after CNS injury.
Copyright © 2015, American Association for the Advancement of Science.
Figures

Comment in
-
Neuroscience. Systemically treating spinal cord injury.Science. 2015 Apr 17;348(6232):285-6. doi: 10.1126/science.aab1615. Epub 2015 Apr 16. Science. 2015. PMID: 25883342 No abstract available.
-
CNS injury: Microtubule stabilizer repairs spinal cord injury.Nat Rev Drug Discov. 2015 May;14(5):310. doi: 10.1038/nrd4616. Epub 2015 Apr 24. Nat Rev Drug Discov. 2015. PMID: 25907343 No abstract available.
References
-
- Klapka N, et al. Suppression of fibrous scarring in spinal cord injury of rat promotes long-distance regeneration of corticospinal tract axons, rescue of primary motoneurons in somatosensory cortex and significant functional recovery. Eur J Neurosci. 2005 Dec;22:3047. - PubMed
-
- GrandPre T, Li S, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547. 05/30/print. - PubMed
-
- Bradbury EJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002 Apr 11;416:636. - PubMed
-
- Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269. 01/18/print. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

