A mechanistic multicentre, parallel group, randomised placebo-controlled trial of mesalazine for the treatment of IBS with diarrhoea (IBS-D)
- PMID: 25765462
- PMCID: PMC4717357
- DOI: 10.1136/gutjnl-2015-309122
A mechanistic multicentre, parallel group, randomised placebo-controlled trial of mesalazine for the treatment of IBS with diarrhoea (IBS-D)
Abstract
Introduction: Immune activation has been reported in the mucosa of IBS patients with diarrhoea (IBS-D), and some small studies have suggested that mesalazine may reduce symptoms. We performed a double-blind, randomised placebo-controlled trial of 2 g mesalazine twice daily versus placebo for 3 months in patients with Rome III criteria IBS-D. Primary outcome was daily average stool frequency during weeks 11-12; secondary outcomes were abdominal pain, stool consistency, urgency and satisfactory relief of IBS symptoms.
Methods: Participants were randomised after a 2-week baseline stool diary. All participants completed a 12-week stool diary and at the end of each week recorded the presence of 'satisfactory relief of IBS symptoms'.
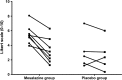
Results: 136 patients with IBS-D (82 women, 54 men) were randomised, 10 patients withdrew from each group. Analysis by intention to treat showed the daily average stool frequency during weeks 11 and 12 were mean (SD), 2.8 (1.2) in mesalazine and 2.7 (1.9) in the placebo group with no significant group difference, (95% CI) 0.1 (-0.33 to 0.53), p=0.66. Mesalazine did not improve abdominal pain, stool consistency nor percentage with satisfactory relief compared with placebo during the last two-weeks follow-up.
Conclusions: This study does not support any clinically meaningful benefit or harm of mesalazine compared with placebo in unselected patients with IBS-D. More precise subtyping based on underlying disease mechanisms is needed to allow more effective targeting of treatment in IBS.
Trial registration number: NCT01316718.
Keywords: 5-AMINOSALICYLIC ACID (5-ASA); DIARRHOEA; IRRITABLE BOWEL SYNDROME.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures

 , telephone visits.
, telephone visits.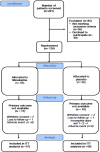

Comment in
-
Efficacy of mesalazine in IBS.Gut. 2016 Jan;65(1):187-8. doi: 10.1136/gutjnl-2015-309593. Epub 2015 Apr 14. Gut. 2016. PMID: 25873641 No abstract available.
-
In search for a disease-modifying treatment in irritable bowel syndrome.Gut. 2016 Jan;65(1):2-3. doi: 10.1136/gutjnl-2015-310024. Epub 2015 Jun 25. Gut. 2016. PMID: 26113178 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
