Characterizing excited conformational states of RNA by NMR spectroscopy
- PMID: 25765780
- PMCID: PMC4755334
- DOI: 10.1016/j.sbi.2015.02.011
Characterizing excited conformational states of RNA by NMR spectroscopy
Abstract
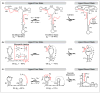
Conformational dynamics is a hallmark of diverse non-coding RNA functions. During these functional processes, RNA molecules almost ubiquitously undergo conformational transitions that are tuned to meet distinct structural and kinetic requirements for proper function. A complete mechanistic understanding of RNA function requires comprehensive structural and dynamic knowledge of these complex transitions, which often involve alternative higher-energy conformational states that pose a major challenge for high-resolution structural study by conventional methods. In this review, we describe recent progress in RNA NMR that has started to unveil detailed structural, thermodynamic and kinetic insights into some of these excited conformational states of RNA and their functional roles in biology.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
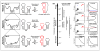

Similar articles
-
Time-resolved NMR spectroscopy: ligand-induced refolding of riboswitches.Methods Mol Biol. 2009;540:161-71. doi: 10.1007/978-1-59745-558-9_12. Methods Mol Biol. 2009. PMID: 19381559
-
Characterizing RNA Excited States Using NMR Relaxation Dispersion.Methods Enzymol. 2015;558:39-73. doi: 10.1016/bs.mie.2015.02.002. Epub 2015 Mar 25. Methods Enzymol. 2015. PMID: 26068737 Free PMC article.
-
Measuring Residual Dipolar Couplings in Excited Conformational States of Nucleic Acids by CEST NMR Spectroscopy.J Am Chem Soc. 2015 Oct 28;137(42):13480-3. doi: 10.1021/jacs.5b09014. Epub 2015 Oct 15. J Am Chem Soc. 2015. PMID: 26462068 Free PMC article.
-
Exploring ribozyme conformational changes with X-ray crystallography.Methods. 2009 Oct;49(2):87-100. doi: 10.1016/j.ymeth.2009.06.003. Epub 2009 Jun 24. Methods. 2009. PMID: 19559088 Free PMC article. Review.
-
Probing invisible, low-populated States of protein molecules by relaxation dispersion NMR spectroscopy: an application to protein folding.Acc Chem Res. 2008 Mar;41(3):442-51. doi: 10.1021/ar700189y. Epub 2008 Feb 15. Acc Chem Res. 2008. PMID: 18275162 Review.
Cited by
-
Shortening the HIV-1 TAR RNA Bulge by a Single Nucleotide Preserves Motional Modes over a Broad Range of Time Scales.Biochemistry. 2016 Aug 16;55(32):4445-56. doi: 10.1021/acs.biochem.6b00285. Epub 2016 Aug 4. Biochemistry. 2016. PMID: 27232530 Free PMC article.
-
Rational design of hairpin RNA excited states reveals multi-step transitions.Nat Commun. 2022 Mar 21;13(1):1523. doi: 10.1038/s41467-022-29194-8. Nat Commun. 2022. PMID: 35314698 Free PMC article.
-
Slow Rotational Dynamics of Cytosine NH2 Groups in Double-Stranded DNA.Biochemistry. 2022 Jul 19;61(14):1415-1418. doi: 10.1021/acs.biochem.2c00299. Epub 2022 Jun 27. Biochemistry. 2022. PMID: 35759792 Free PMC article.
-
Base-Pair Opening Dynamics Study of Fluoride Riboswitch in the Bacillus cereus CrcB Gene.Int J Mol Sci. 2021 Mar 22;22(6):3234. doi: 10.3390/ijms22063234. Int J Mol Sci. 2021. PMID: 33810132 Free PMC article.
-
Chemical shift prediction of RNA imino groups: application toward characterizing RNA excited states.Nat Commun. 2021 Mar 11;12(1):1595. doi: 10.1038/s41467-021-21840-x. Nat Commun. 2021. PMID: 33707433 Free PMC article.
References
-
- Kruger K, Grabowski PJ, Zaug AJ, Sands J, Gottschling DE, Cech TR. Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell. 1982;31:147–157. - PubMed
-
- Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983;35:849–857. - PubMed
-
- Mattick JS. RNA regulation: a new genetics? Nat Rev Genet. 2004;5:316–323. - PubMed
-
- Sharp PA. The centrality of RNA. Cell. 2009;136:577–580. - PubMed
-
- Williamson JR. Induced fit in RNA–protein recognition. Nat Struct Biol. 2000;7:834–837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

