HIV cell-to-cell transmission: effects on pathogenesis and antiretroviral therapy
- PMID: 25766144
- PMCID: PMC4417442
- DOI: 10.1016/j.tim.2015.02.003
HIV cell-to-cell transmission: effects on pathogenesis and antiretroviral therapy
Abstract
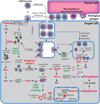
HIV spreads more efficiently in vitro when infected cells directly contact uninfected cells to form virological synapses. A hallmark of virological synapses is that viruses can be transmitted at a higher multiplicity of infection (MOI) that, in vitro, results in a higher number of proviruses. Whether HIV also spreads by cell-cell contact in vivo is a matter of debate. Here we discuss recent data that suggest that contact-mediated transmission largely manifests itself in vivo as CD4+ T cell depletion. The assault of a cell by a large number of incoming particles is likely to be efficiently sensed by the innate cellular surveillance to trigger cell death. The large number of particles transferred across virological synapses has also been implicated in reduced efficacy of antiretroviral therapies. Thus, antiretroviral therapies must remain effective against the high MOI observed during cell-to-cell transmission to inhibit both viral replication and the pathogenesis associated with HIV infection.
Keywords: antiretroviral therapy; cell-to-cell transmission; human immunodeficiency virus; virological synapse.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures



References
-
- Sattentau Q. Avoiding the void: cell-to-cell spread of human viruses. Nat. Rev. Microbiol. 2008;6:815–826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

