Targeting FcRn for the modulation of antibody dynamics
- PMID: 25766596
- PMCID: PMC4529761
- DOI: 10.1016/j.molimm.2015.02.007
Targeting FcRn for the modulation of antibody dynamics
Abstract
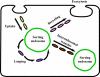
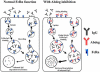
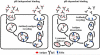
The MHC class I-related receptor, FcRn, is a multitasking protein that transports its IgG ligand within and across cells of diverse origins. The role of this receptor as a global regulator of IgG homeostasis and transport, combined with knowledge of the molecular details of FcRn-IgG interactions, has led to opportunities to modulate the in vivo dynamics of antibodies and their antigens through protein engineering. Consequently, the generation of half-life extended antibodies has shown a rapid expansion over the past decade. Further, FcRn itself can be targeted by inhibitors to induce decreased levels of circulating IgGs, which could have applications in multiple clinical settings. The engineering of antibody-antigen interactions to reduce antibody-mediated buffering of soluble ligand has also developed into an active area of investigation, leading to novel antibody platforms designed to result in more effective antigen clearance. Similarly, the target-mediated elimination of antibodies by internalizing, membrane bound antigens (receptors) can be decreased using novel engineering approaches. These strategies, combined with subcellular trafficking analyses of antibody/antigen/FcRn behavior in cells to predict in vivo behavior, have considerable promise for the production of next generation therapeutics and diagnostics.
Keywords: Antibody engineering; FcRn; IgG; Pharmacokinetics; Therapeutic antibodies; pH-dependence.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

References
-
- Adamski FM, et al. Expression of the Fc receptor in the mammary gland during lactation in the marsupial Trichosurus vulpecula (brushtail possum) Mol. Immunol. 2000;37:435–444. - PubMed
-
- Ahouse JJ, et al. Mouse MHC class I-like Fc receptor encoded outside the MHC. J. Immunol. 1993;151:6076–6088. - PubMed
-
- Akilesh S, et al. Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J. Immunol. 2007;179:4580–4588. - PubMed
-
- Andersen JT, et al. The conserved histidine 166 residue of the human neonatal Fc receptor heavy chain is critical for the pH-dependent binding to albumin. Eur. J. Immunol. 2006;36:3044–3051. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

