Glia and alpha-synuclein in neurodegeneration: A complex interaction
- PMID: 25766679
- PMCID: PMC4730552
- DOI: 10.1016/j.nbd.2015.03.003
Glia and alpha-synuclein in neurodegeneration: A complex interaction
Abstract
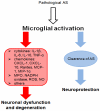
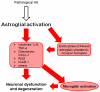
α-Synucleinopathies (ASP) comprise adult-onset, progressive neurodegenerative disorders such as Parkinson's disease (PD), dementia with Lewy bodies (DLB) and multiple system atrophy (MSA) that are characterized by α-synuclein (AS) aggregates in neurons or glia. PD and DLB feature neuronal AS-positive inclusions termed Lewy bodies (LB) whereas glial cytoplasmic inclusions (GCIs, Papp-Lantos bodies) are recognized as the defining hallmark of MSA. Furthermore, AS-positive cytoplasmic aggregates may also be seen in astroglial cells of PD/DLB and MSA brains. The glial AS-inclusions appear to trigger reduced trophic support resulting in neuronal loss. Moreover, microgliosis and astrogliosis can be found throughout the neurodegenerative brain and both are key players in the initiation and progression of ASP. In this review, we will highlight AS-dependent alterations of glial function and their impact on neuronal vulnerability thereby providing a detailed summary on the multifaceted role of glia in ASP.
Keywords: Astroglia; Dementia with Lewy bodies; Glial cytoplasmic inclusions; Lewy bodies; Microglia; Multiple system atrophy; Oligodendroglia; Parkinson's disease; α-Synuclein.
Copyright © 2015. Published by Elsevier Inc.
Figures
References
-
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25(1):239–252. - PubMed
-
- Aerts MB, Esselink RA, Abdo WF, Bloem BR, Verbeek MM. CSF alpha-synuclein does not differentiate between parkinsonian disorders. Neurobiol Aging. 2012;33(2):430, e431–433. - PubMed
-
- Ahmed Z, Asi YT, Sailer A, Lees AJ, Houlden H, Revesz T, Holton JL. The neuropathology, pathophysiology and genetics of multiple system atrophy. Neuropathol Appl Neurobiol. 2012;38(1):4–24. - PubMed
-
- Al-Chalabi A, Durr A, Wood NW, Parkinson MH, Camuzat A, Hulot JS, Morrison KE, Renton A, Sussmuth SD, Landwehrmeyer BG, Ludolph A, Agid Y, Brice A, Leigh PN, Bensimon G, Group, N. G. S. Genetic variants of the alpha-synuclein gene SNCA are associated with multiple system atrophy. PLoS One. 2009;4(9):e7114. - PMC - PubMed
-
- Alvarez-Erviti L, Couch Y, Richardson J, Cooper JM, Wood MJ. Alpha-synuclein release by neurons activates the inflammatory response in a microglial cell line. Neurosci Res. 2011;69(4):337–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

