Phase 3 study of nilotinib vs imatinib in Chinese patients with newly diagnosed chronic myeloid leukemia in chronic phase: ENESTchina
- PMID: 25766724
- PMCID: PMC4416528
- DOI: 10.1182/blood-2014-09-601674
Phase 3 study of nilotinib vs imatinib in Chinese patients with newly diagnosed chronic myeloid leukemia in chronic phase: ENESTchina
Abstract
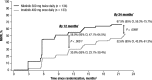
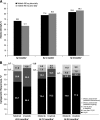
Treatment with a tyrosine kinase inhibitor (TKI) targeting BCR-ABL1 is currently the standard of care for patients with chronic myeloid leukemia (CML) in chronic phase (CML-CP). In this study, we present results of the ENESTchina (Evaluating Nilotinib Efficacy and Safety in Clinical Trials-China) that was conducted to investigate nilotinib 300 mg twice daily vs imatinib 400 mg once daily in a Chinese population. ENESTchina met its primary end point with a statistically significant higher rate of major molecular response (MMR; BCR-ABL1 ≤0.1% on the International Scale) at 12 months in the nilotinib arm vs the imatinib arm (52.2% vs 27.8%; P < .0001), and MMR rates remained higher with nilotinib vs imatinib throughout the follow-up period. Rates of complete cytogenetic response (0% Philadelphia chromosome-positive [Ph+] metaphases by standard cytogenetics) were comparable and ≥80% by 24 months in both arms. The estimated rate of freedom from progression to accelerated phase/blast crisis at 24 months was 95.4% in each arm. The safety profiles of both drugs were similar to those from previous studies. In conclusion, rates of MMR at 12 months were superior with nilotinib vs imatinib in Chinese patients with newly diagnosed Ph+ CML-CP. This trial was registered at www.clinicaltrials.gov as #NCT01275196.
© 2015 by The American Society of Hematology.
Figures
References
-
- Tasigna(R) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2014.
-
- Gleevec(R) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
-
- Saglio G, Kim DW, Issaragrisil S, et al. ENESTnd Investigators. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–2259. - PubMed
-
- Kantarjian HM, Hochhaus A, Saglio G, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12(9):841–851. - PubMed
-
- Larson RA, Hochhaus A, Hughes TP, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26(10):2197–2203. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

