Novel model-based dosing guidelines for gentamicin and tobramycin in preterm and term neonates
- PMID: 25766737
- PMCID: PMC4472325
- DOI: 10.1093/jac/dkv052
Novel model-based dosing guidelines for gentamicin and tobramycin in preterm and term neonates
Abstract
Objectives: In the heterogeneous group of preterm and term neonates, gentamicin and tobramycin are mainly dosed according to empirical guidelines, after which therapeutic drug monitoring and subsequent dose adaptation are applied. In view of the variety of neonatal guidelines available, the purpose of this study was to evaluate target concentration attainment of these guidelines, and to propose a new model-based dosing guideline for these drugs in neonates.
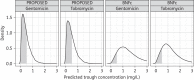
Methods: Demographic characteristics of 1854 neonates (birth weight 390-5200 g, post-natal age 0-27 days) were extracted from earlier studies and sampled to obtain a test dataset of 5000 virtual patients. Monte Carlo simulations on the basis of validated models were undertaken to evaluate the attainment of target peak (5-12 mg/L) and trough (<0.5 mg/L) concentrations, and cumulative AUC, with the existing and proposed guidelines.
Results: Across the entire neonatal age and weight range, the Dutch National Formulary for Children, the British National Formulary for Children, Neofax and the Red Book resulted in adequate peak but elevated trough concentrations (63%-90% above target). The proposed dosing guideline (4.5 mg/kg gentamicin or 5.5 mg/kg tobramycin) with a dosing interval based on birth weight and post-natal age leads to adequate peak concentrations with only 33%-38% of the trough concentrations above target, and a constant AUC across weight and post-natal age.
Conclusions: The proposed neonatal dosing guideline for gentamicin and tobramycin results in improved attainment of target concentrations and should be prospectively evaluated in clinical studies to evaluate the efficacy and safety of this treatment.
Keywords: NONMEM; aminoglycosides; pharmacokinetics; therapeutic drug monitoring.
© The Author 2015. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Dutch Knowledge Centre for Pharmacotherapy in Children. Dutch National Formulary for Children/Kinderformularium. http://www.kinderformularium.nl/.
-
- Paediatric Formulary Committee. British National Formulary for Children. London: BMJ Group, 2009.
-
- Pickering LK, Baker CJ, Kimberlin DW. Red Book: 2012 Report of the Committee on Infectious Diseases. American Academy of Pediatrics, 2012.
-
- Young TE. Neofax. Montvale: Thomson Reuters, 2011.
-
- De Hoog M, Schoemaker RC, Mouton JW, et al. Tobramycin population pharmacokinetics in neonates. Clin Pharmacol Ther 1997; 62: 392–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

