Attention Stabilizes Representations in the Human Hippocampus
- PMID: 25766839
- PMCID: PMC4712804
- DOI: 10.1093/cercor/bhv041
Attention Stabilizes Representations in the Human Hippocampus
Abstract
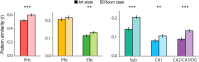
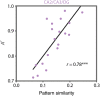
Attention and memory are intricately linked, but how attention modulates brain areas that subserve memory, such as the hippocampus, is unknown. We hypothesized that attention may stabilize patterns of activity in human hippocampus, resulting in distinct but reliable activity patterns for different attentional states. To test this prediction, we utilized high-resolution functional magnetic resonance imaging and a novel "art gallery" task. On each trial, participants viewed a room containing a painting, and searched a stream of rooms for a painting from the same artist (art state) or a room with the same layout (room state). Bottom-up stimulation was the same in both tasks, enabling the isolation of neural effects related to top-down attention. Multivariate analyses revealed greater pattern similarity in all hippocampal subfields for trials from the same, compared with different, attentional state. This stability was greater for the room than art state, was unrelated to univariate activity, and, in CA2/CA3/DG, was correlated with behavior. Attention therefore induces representational stability in the human hippocampus, resulting in distinct activity patterns for different attentional states. Modulation of hippocampal representational stability highlights the far-reaching influence of attention outside of sensory systems.
Keywords: attentional modulation; high-resolution fMRI; hippocampal subfields; medial temporal lobe; task representations.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


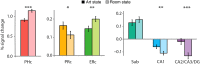
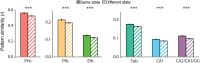

References
-
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE. 2006. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 50:507–517. - PubMed
-
- Brown MW, Aggleton JP. 2001. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2:51–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

