Distinct pathways regulate Syk protein activation downstream of immune tyrosine activation motif (ITAM) and hemITAM receptors in platelets
- PMID: 25767114
- PMCID: PMC4416859
- DOI: 10.1074/jbc.M114.629527
Distinct pathways regulate Syk protein activation downstream of immune tyrosine activation motif (ITAM) and hemITAM receptors in platelets
Abstract
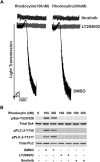
Tyrosine kinase pathways are known to play an important role in the activation of platelets. In particular, the GPVI and CLEC-2 receptors are known to activate Syk upon tyrosine phosphorylation of an immune tyrosine activation motif (ITAM) and hemITAM, respectively. However, unlike GPVI, the CLEC-2 receptor contains only one tyrosine motif in the intracellular domain. The mechanisms by which this receptor activates Syk are not completely understood. In this study, we identified a novel signaling mechanism in CLEC-2-mediated Syk activation. CLEC-2-mediated, but not GPVI-mediated, platelet activation and Syk phosphorylation were abolished by inhibition of PI3K, which demonstrates that PI3K regulates Syk downstream of CLEC-2. Ibrutinib, a Tec family kinase inhibitor, also completely abolished CLEC-2-mediated aggregation and Syk phosphorylation in human and murine platelets. Furthermore, embryos lacking both Btk and Tec exhibited cutaneous edema associated with blood-filled vessels in a typical lymphatic pattern similar to CLEC-2 or Syk-deficient embryos. Thus, our data show, for the first time, that PI3K and Tec family kinases play a crucial role in the regulation of platelet activation and Syk phosphorylation downstream of the CLEC-2 receptor.
Keywords: Cell Signaling; Platelet; Signal Transduction; Signaling; Thrombosis.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures








Similar articles
-
The novel Syk inhibitor R406 reveals mechanistic differences in the initiation of GPVI and CLEC-2 signaling in platelets.J Thromb Haemost. 2009 Jul;7(7):1192-9. doi: 10.1111/j.1538-7836.2009.03451.x. Epub 2009 Apr 24. J Thromb Haemost. 2009. PMID: 19422460
-
The N-terminal SH2 domain of Syk is required for (hem)ITAM, but not integrin, signaling in mouse platelets.Blood. 2015 Jan 1;125(1):144-54. doi: 10.1182/blood-2014-05-579375. Epub 2014 Oct 28. Blood. 2015. PMID: 25352128 Free PMC article.
-
Phosphorylation on Syk Y342 is important for both ITAM and hemITAM signaling in platelets.J Biol Chem. 2022 Aug;298(8):102189. doi: 10.1016/j.jbc.2022.102189. Epub 2022 Jun 24. J Biol Chem. 2022. PMID: 35753354 Free PMC article.
-
Platelet immunoreceptor tyrosine-based activation motif (ITAM) and hemITAM signaling and vascular integrity in inflammation and development.J Thromb Haemost. 2016 Apr;14(4):645-54. doi: 10.1111/jth.13250. Epub 2016 Feb 16. J Thromb Haemost. 2016. PMID: 26749528 Review.
-
GPVI and CLEC-2 in hemostasis and vascular integrity.J Thromb Haemost. 2010 Jul;8(7):1456-67. doi: 10.1111/j.1538-7836.2010.03875.x. Epub 2010 Mar 25. J Thromb Haemost. 2010. PMID: 20345705 Review.
Cited by
-
Platelet CLEC-2 and lung development.Res Pract Thromb Haemost. 2020 Apr 19;4(4):481-490. doi: 10.1002/rth2.12338. eCollection 2020 May. Res Pract Thromb Haemost. 2020. PMID: 32548549 Free PMC article. Review.
-
The Activating C-type Lectin-like Receptor NKp65 Signals through a Hemi-immunoreceptor Tyrosine-based Activation Motif (hemITAM) and Spleen Tyrosine Kinase (Syk).J Biol Chem. 2017 Feb 24;292(8):3213-3223. doi: 10.1074/jbc.M116.759977. Epub 2017 Jan 12. J Biol Chem. 2017. PMID: 28082678 Free PMC article.
-
Role of Liver Kinase 1B in Platelet Activation and Host Defense During Klebsiella pneumoniae-Induced Pneumosepsis.Int J Mol Sci. 2025 Apr 14;26(8):3714. doi: 10.3390/ijms26083714. Int J Mol Sci. 2025. PMID: 40332331 Free PMC article.
-
Assessment of thrombotic risk during long-term treatment of immune thrombocytopenia with fostamatinib.Ther Adv Hematol. 2021 Apr 30;12:20406207211010875. doi: 10.1177/20406207211010875. eCollection 2021. Ther Adv Hematol. 2021. PMID: 33995988 Free PMC article.
-
Megakaryocyte-specific knockout of the Mir-99b/let7e/125a cluster lowers platelet count without altering platelet function.Blood Cells Mol Dis. 2021 Dec;92:102624. doi: 10.1016/j.bcmd.2021.102624. Epub 2021 Nov 3. Blood Cells Mol Dis. 2021. PMID: 34775219 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

