Quantitative Description of a Protein Fitness Landscape Based on Molecular Features
- PMID: 25767204
- PMCID: PMC4476158
- DOI: 10.1093/molbev/msv059
Quantitative Description of a Protein Fitness Landscape Based on Molecular Features
Abstract
Understanding the driving forces behind protein evolution requires the ability to correlate the molecular impact of mutations with organismal fitness. To address this issue, we employ here metallo-β-lactamases as a model system, which are Zn(II) dependent enzymes that mediate antibiotic resistance. We present a study of all the possible evolutionary pathways leading to a metallo-β-lactamase variant optimized by directed evolution. By studying the activity, stability and Zn(II) binding capabilities of all mutants in the preferred evolutionary pathways, we show that this local fitness landscape is strongly conditioned by epistatic interactions arising from the pleiotropic effect of mutations in the different molecular features of the enzyme. Activity and stability assays in purified enzymes do not provide explanatory power. Instead, measurement of these molecular features in an environment resembling the native one provides an accurate description of the observed antibiotic resistance profile. We report that optimization of Zn(II) binding abilities of metallo-β-lactamases during evolution is more critical than stabilization of the protein to enhance fitness. A global analysis of these parameters allows us to connect genotype with fitness based on quantitative biochemical and biophysical parameters.
Keywords: antibiotic resistance; epistasis; metallo-ß-lactamase; protein evolution.
© The Author 2015. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


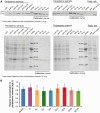
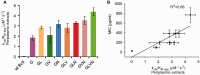

References
-
- Bershtein S, Goldin K, Tawfik DS. Intense neutral drifts yield robust and evolvable consensus proteins. J Mol Biol. 2008;379:1029–1044. - PubMed
-
- Bershtein S, Segal M, Bekerman R, Tokuriki N, Tawfik DS. Robustness-epistasis link shapes the fitness landscape of a randomly drifting protein. Nature. 2006;444:929–932. - PubMed
-
- Breen MS, Kemena C, Vlasov PK, Notredame C, Kondrashov FA. Epistasis as the primary factor in molecular evolution. Nature. 2012;490:535–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

