Bioadhesive films containing fluconazole for mucocutaneous candidiasis
- PMID: 25767319
- PMCID: PMC4355883
- DOI: 10.4103/0250-474x.151601
Bioadhesive films containing fluconazole for mucocutaneous candidiasis
Abstract
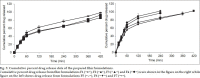
Fluconazole is a broad spectrum antifungal agent that has been extensively applied for the management of oral, pharyngeal and cutaneous candidiasis. Fluconazole has a high volume of distribution (0.55-0.65 l/kg) and has systemic toxicity due to high drug-drug interaction. The present study focuses on the formulation of bioadhesive film as a controlled release carrier for fluconazole. The formulation was intended to provide localized delivery of fluconazole exclusively at the site of infection, thereby reducing its total dose and hence, dose-related toxicities. Bioadhesive films were prepared by solvent casting method using sodium alginate and polyvinyl alcohol alone as well as in various combinations. Prepared films were evaluated for physical characteristics like, weight and content uniformity, film thickness, swelling index, microenvironment pH and folding endurance. In vitro drug release, in vitro and ex vivo residence time, bioadhesive strength and skin irritation were also studied. Accelerated stability study was conducted on the optimized formulation as per ICH guidelines. Weight of all the films were not more than 20 mg. Thickness of the films ranged between 0.09 to 0.15 mm whereas swelling indices showed a high extent of variation. Films composed of polyvinyl alcohol alone provided a swelling index of 6%. Bioadhesive strength was found to be more than 18 g. Microenvironment pH was near to 7.0 for most of the formulations. Ex vivo residence time of optimized batch was more than 5 h and it provided controlled drug release up to 8 h. As revealed in FT-IR and DSC studies, drug was found to be compatible with the excipients used in this study.
Keywords: Fluconzole; bioadhesive film; localized delivery; mucocutaneous candidiasis.
Figures
Similar articles
-
Efficacy and Safety of Fluconazole Mucoadhesive Patches in Human Immunodeficiency Virus-Related Oral Candidiasis.J Int Assoc Provid AIDS Care. 2024 Jan-Dec;23:23259582241299014. doi: 10.1177/23259582241299014. J Int Assoc Provid AIDS Care. 2024. PMID: 39632750 Free PMC article. Clinical Trial.
-
Fluconazole mucoadhesive buccal films: in vitro/in vivo performance.Curr Drug Deliv. 2009 Jan;6(1):17-27. doi: 10.2174/156720109787048195. Curr Drug Deliv. 2009. PMID: 19418952
-
Formulation, development and characterization of mucoadhesive film for treatment of vaginal candidiasis.Int J Pharm Investig. 2016 Jan-Mar;6(1):47-55. doi: 10.4103/2230-973X.176487. Int J Pharm Investig. 2016. PMID: 27014619 Free PMC article.
-
Design of novel miconazole nitrate transdermal films based on Eudragit RS100 and HPMC hybrids: preparation, physical characterization, in vitro and ex vivo studies.Drug Deliv. 2015 Dec;22(8):1078-1085. doi: 10.3109/10717544.2013.875604. Epub 2014 Jan 23. Drug Deliv. 2015. PMID: 24455998
-
Fast Dissolving Sublingual Films of Ondansetron Hydrochloride: Effect of Additives on in vitro Drug Release and Mucosal Permeation.J Young Pharm. 2010 Jul;2(3):216-22. doi: 10.4103/0975-1483.66790. J Young Pharm. 2010. PMID: 21042474 Free PMC article.
Cited by
-
Investigating the performance of drug delivery system of fluconazole made of nano-micro fibers coated on cotton/polyester fabric.J Mater Sci Mater Med. 2017 Sep 27;28(11):175. doi: 10.1007/s10856-017-5957-9. J Mater Sci Mater Med. 2017. PMID: 28956211
-
Preparation and In Vitro Evaluation of Alginate Microparticles Containing Amphotericin B for the Treatment of Candida Infections.Int J Biomater. 2020 Aug 1;2020:2514387. doi: 10.1155/2020/2514387. eCollection 2020. Int J Biomater. 2020. PMID: 32802065 Free PMC article.
-
Antibacterial and Cellular Response Toward a Gasotransmitter-Based Hybrid Wound Dressing.ACS Biomater Sci Eng. 2019 Aug 12;5(8):4002-4012. doi: 10.1021/acsbiomaterials.9b00737. Epub 2019 Jul 17. ACS Biomater Sci Eng. 2019. PMID: 33443422 Free PMC article.
-
Miconazole-Urea in a Buccal Film as a New Trend for Treatment of Resistant Mouth Fungal White Patches.Front Microbiol. 2018 May 11;9:837. doi: 10.3389/fmicb.2018.00837. eCollection 2018. Front Microbiol. 2018. PMID: 29867789 Free PMC article.
-
Potential Use of Alginate-Based Carriers As Antifungal Delivery System.Front Microbiol. 2017 Jan 30;8:97. doi: 10.3389/fmicb.2017.00097. eCollection 2017. Front Microbiol. 2017. PMID: 28194145 Free PMC article. Review.
References
-
- Murray PA, Koletar SL, Mallegol I, Wu J, Moskovitz BL. Itraconazole oral solution versus clotrimazole troches for the treatment of oropharyngeal candidiasis in immunocompromised patients. Clin Ther. 1997;19:471–80. - PubMed
-
- Bachhav YG, Patravale VB. Microemulsion-based Vaginal Gel of Fluconazole: Formulation, In vitro and In vivo Evaluation. Int J Pharm. 2009;365:175–9. - PubMed
-
- Meis J, Petrou M, Bille J, Ellis D, Gibbs D. A global evaluation of the susceptibility of candida species to fluconazole by disk diffusion. Diagn Microbiol Infect Dis. 2000;36:215–23. - PubMed
-
- Mohan H. 5th ed. New Delhi: Jaypee Brothers, Medical Publishers; 2005. Textbook of pathology; pp. 188–90.
LinkOut - more resources
Full Text Sources
Research Materials