Effects of parecoxib on analgesia benefit and blood loss following open prostatectomy: a multicentre randomized trial
- PMID: 25767411
- PMCID: PMC4357198
- DOI: 10.1186/s12871-015-0015-y
Effects of parecoxib on analgesia benefit and blood loss following open prostatectomy: a multicentre randomized trial
Abstract
Background: This multi-centre, prospective, randomized, double-blind, placebo-controlled study was designed to test the hypotheses that parecoxib improves patients' postoperative analgesia without increasing surgical blood loss following radical open prostatectomy.
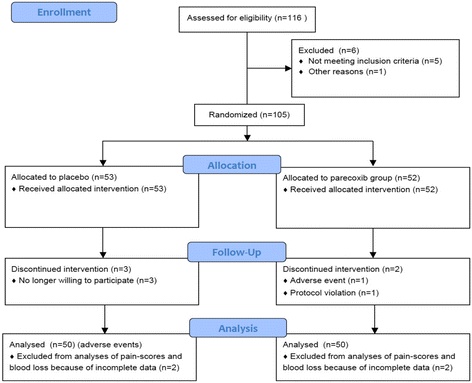
Methods: 105 patients (64 ± 7 years old) were randomized to receive either parecoxib or placebo with concurrent morphine patient controlled analgesia. Cumulative opioid consumption (primary objective) and the overall benefit of analgesia score (OBAS), the modified brief pain inventory short form (m-BPI-sf), the opioid-related symptom distress scale (OR-SDS), and perioperative blood loss (secondary objectives) were assessed.
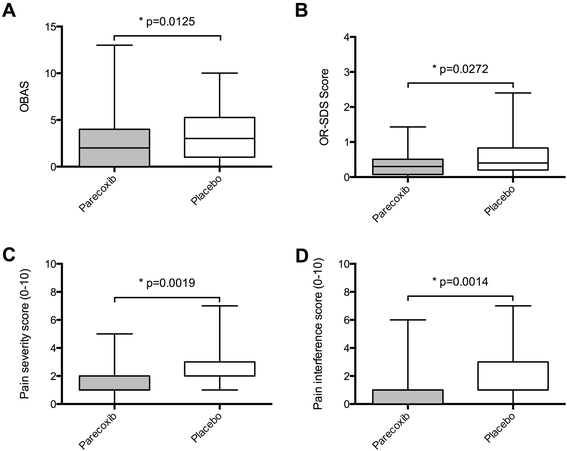
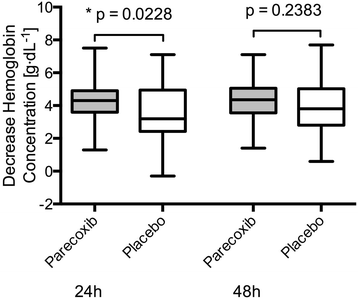
Results: In each group 48 patients received the study medication for 48 hours postoperatively. Parecoxib significantly reduced cumulative opioid consumption by 24% (43 ± 24.1 mg versus 57 ± 28 mg, mean ± SD, p=0.02), translating into improved benefit of analgesia (OBAS: 2(0/4) versus 3(1/5.25), p=0.01), pain severity (m-BPI-sf: 1(1/2) versus 2(2/3), p < 0.01) and pain interference (m-BPI-sf: 1(0/1) versus 1(1/3), p=0.001), as well as reduced opioid-related side effects (OR-SDS score: 0.3(0.075/0.51) versus 0.4(0.2/0.83), p=0.03). Blood loss was significantly higher at 24 hours following surgery in the parecoxib group (4.3 g⋅dL(-1) (3.6/4.9) versus (3.2 g⋅dL(-1) (2.4/4.95), p=0.02).
Conclusions: Following major abdominal surgery, parecoxib significantly improves patients' perceived analgesia. Parecoxib may however increase perioperative blood loss. Further trials are needed to evaluate the effects of selective cyclooxygenase-2 inhibitors on blood loss.
Trial registration: ClinicalTrials.gov Identifier: NCT00346268.
Keywords: Analgesics non-opioids; Analgesics opioids; Morphine; Pain; Parecoxib; Postoperative pain.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

