Theranostic impact of NG2/CSPG4 proteoglycan in cancer
- PMID: 25767619
- PMCID: PMC4350014
- DOI: 10.7150/thno.10824
Theranostic impact of NG2/CSPG4 proteoglycan in cancer
Abstract
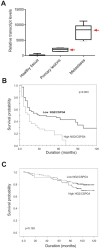
NG2/CSPG4 is an unusual cell-membrane integral proteoglycan widely recognized to be a prognostic factor, a valuable tool for ex vivo and non-invasive molecular diagnostics and, by virtue of its tight association with malignancy, a tantalizing therapeutic target in several tumour types. Although the biology behind its involvement in cancer progression needs to be better understood, implementation of NG2/CSPG4 in the routine clinical practice is attainable and has the potential to contribute to an improved individualized management of cancer patients. In this context, its polymorphic nature seems to be particularly valuable in the effort to standardize informative diagnostic procedures and consolidate forcible immunotherapeutic treatment strategies. We discuss here the underpinnings for this potential and highlight the benefits of taking advantage of the intra-tumour and inter-patient variability in the regulation of NG2/CSPG4 expression. We envision that NG2/CSPG4 may effectively be exploited in therapeutic interventions aimed at averting resistance to target therapy agents and at interfering with secondary lesion formation and/or tumour recurrence.
Keywords: CSPG4; NG2; cancer; proteoglycan.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Chekenya M, Hjelstuen M, Enger P O. et al. NG2 proteoglycan promotes angiogenesis-dependent tumor growth in CNS by sequestering angiostatin. FASEB J. 2002;16:586–588. - PubMed
-
- Campoli M R, Chang C C, Kageshita T, Wang X, McCarthy J B, Ferrone S. Human high molecular weight-melanoma-associated antigen (HMW-MAA): a melanoma cell surface chondroitin sulfate proteoglycan (MSCP) with biological and clinical significance. Crit Rev Immunol. 2004;24:267–296. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

