Evolution of alternative biosynthetic pathways for vitamin C following plastid acquisition in photosynthetic eukaryotes
- PMID: 25768426
- PMCID: PMC4396506
- DOI: 10.7554/eLife.06369
Evolution of alternative biosynthetic pathways for vitamin C following plastid acquisition in photosynthetic eukaryotes
Abstract
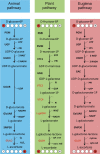
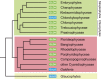
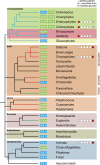
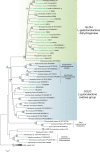
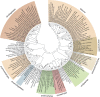
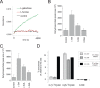
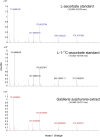
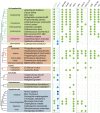
Ascorbic acid (vitamin C) is an enzyme co-factor in eukaryotes that also plays a critical role in protecting photosynthetic eukaryotes against damaging reactive oxygen species derived from the chloroplast. Many animal lineages, including primates, have become ascorbate auxotrophs due to the loss of the terminal enzyme in their biosynthetic pathway, L-gulonolactone oxidase (GULO). The alternative pathways found in land plants and Euglena use a different terminal enzyme, L-galactonolactone dehydrogenase (GLDH). The evolutionary processes leading to these differing pathways and their contribution to the cellular roles of ascorbate remain unclear. Here we present molecular and biochemical evidence demonstrating that GULO was functionally replaced with GLDH in photosynthetic eukaryote lineages following plastid acquisition. GULO has therefore been lost repeatedly throughout eukaryote evolution. The formation of the alternative biosynthetic pathways in photosynthetic eukaryotes uncoupled ascorbate synthesis from hydrogen peroxide production and likely contributed to the rise of ascorbate as a major photoprotective antioxidant.
Keywords: Galdieria; L-gulonolactone oxidase; Porphyra; ascorbate; evolutionary biology; genomics; plant biology; vitamin C.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures

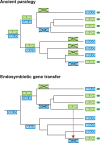
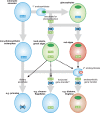
Comment in
-
A cross-kingdom history.Elife. 2015 Apr 15;4:e07527. doi: 10.7554/eLife.07527. Elife. 2015. PMID: 25872909 Free PMC article.
References
-
- Badejo AA, Wada K, Gao Y, Maruta T, Sawa Y, Shigeoka S, Ishikawa T. Translocation and the alternative D-galacturonate pathway contribute to increasing the ascorbate level in ripening tomato fruits together with the D-mannose/L-galactose pathway. Journal of Experimental Botany. 2012;63:229–239. doi: 10.1093/jxb/err275. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

