Individualized homeopathic treatment and fluoxetine for moderate to severe depression in peri- and postmenopausal women (HOMDEP-MENOP study): a randomized, double-dummy, double-blind, placebo-controlled trial
- PMID: 25768800
- PMCID: PMC4359147
- DOI: 10.1371/journal.pone.0118440
Individualized homeopathic treatment and fluoxetine for moderate to severe depression in peri- and postmenopausal women (HOMDEP-MENOP study): a randomized, double-dummy, double-blind, placebo-controlled trial
Erratum in
-
Correction: Individualized Homeopathic Treatment and Fluoxetine for Moderate to Severe Depression in Peri- and Postmenopausal Women (HOMDEP-MENOP Study): A Randomized, Double-Dummy, Double-Blind, Placebo-Controlled Trial.PLoS One. 2015 May 4;10(5):e0127719. doi: 10.1371/journal.pone.0127719. eCollection 2015. PLoS One. 2015. PMID: 25938465 Free PMC article. No abstract available.
Retraction in
-
Retraction: Individualized Homeopathic Treatment and Fluoxetine for Moderate to Severe Depression in Peri- and Postmenopausal Women (HOMDEP-MENOP Study): A Randomized, Double-Dummy, Double-Blind, Placebo-Controlled Trial.PLoS One. 2020 Apr 23;15(4):e0232415. doi: 10.1371/journal.pone.0232415. eCollection 2020. PLoS One. 2020. PMID: 32324840 Free PMC article. No abstract available.
Abstract
Background: Perimenopausal period refers to the interval when women's menstrual cycles become irregular and is characterized by an increased risk of depression. Use of homeopathy to treat depression is widespread but there is a lack of clinical trials about its efficacy in depression in peri- and postmenopausal women. The aim of this study was to assess efficacy and safety of individualized homeopathic treatment versus placebo and fluoxetine versus placebo in peri- and postmenopausal women with moderate to severe depression.
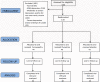
Methods/design: A randomized, placebo-controlled, double-blind, double-dummy, superiority, three-arm trial with a 6 week follow-up study was conducted. The study was performed in a public research hospital in Mexico City in the outpatient service of homeopathy. One hundred thirty-three peri- and postmenopausal women diagnosed with major depression according to DSM-IV (moderate to severe intensity) were included. The outcomes were: change in the mean total score among groups on the 17-item Hamilton Rating Scale for Depression, Beck Depression Inventory and Greene Scale, after 6 weeks of treatment, response and remission rates, and safety. Efficacy data were analyzed in the intention-to-treat population (ANOVA with Bonferroni post-hoc test).
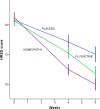
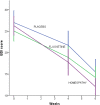
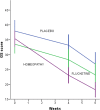
Results: After a 6-week treatment, homeopathic group was more effective than placebo by 5 points in Hamilton Scale. Response rate was 54.5% and remission rate, 15.9%. There was a significant difference among groups in response rate definition only, but not in remission rate. Fluoxetine-placebo difference was 3.2 points. No differences were observed among groups in the Beck Depression Inventory. Homeopathic group was superior to placebo in Greene Climacteric Scale (8.6 points). Fluoxetine was not different from placebo in Greene Climacteric Scale.
Conclusion: Homeopathy and fluoxetine are effective and safe antidepressants for climacteric women. Homeopathy and fluoxetine were significantly different from placebo in response definition only. Homeopathy, but not fluoxetine, improves menopausal symptoms scored by Greene Climacteric Scale.
Trial registration: ClinicalTrials.gov NCT01635218.
Protocol publication: https://clinicaltrials.gov/ct2/show/NCT01635218 [corrected].
Conflict of interest statement
Figures
Comment in
-
Homeopathy for Depression, Music for Postoperative Recovery, Red Yeast Rice for High Cholesterol, Acupuncture for Seasonal Allergic Rhinitis, and Ginger for Osteoarthritis.Explore (NY). 2016 Jul-Aug;12(4):287-91. doi: 10.1016/j.explore.2016.04.015. Epub 2016 Apr 20. Explore (NY). 2016. PMID: 27234467 No abstract available.
-
Individualized Homeopathy for Depression in Climacteric Women: Comments on the Retraction by PLoS ONE.Homeopathy. 2020 Nov;109(4):267-270. doi: 10.1055/s-0040-1714741. Epub 2020 Aug 10. Homeopathy. 2020. PMID: 32777857
Similar articles
-
Efficacy of individualized homeopathic treatment and fluoxetine for moderate to severe depression in peri- and postmenopausal women (HOMDEP-MENOP): study protocol for a randomized, double-dummy, double-blind, placebo-controlled trial.Trials. 2013 Apr 23;14:105. doi: 10.1186/1745-6215-14-105. Trials. 2013. PMID: 23782520 Free PMC article. Clinical Trial.
-
Is metabolic dysregulation associated with antidepressant response in depressed women in climacteric treated with individualized homeopathic medicines or fluoxetine? The HOMDEP-MENOP Study.Homeopathy. 2017 Feb;106(1):3-10. doi: 10.1016/j.homp.2016.11.002. Epub 2017 Jan 10. Homeopathy. 2017. PMID: 28325221 Clinical Trial.
-
Response to Individualized Homeopathic Treatment for Depression in Climacteric Women with History of Domestic Violence, Marital Dissatisfaction or Sexual Abuse: Results from the HOMDEP-MENOP Study.Homeopathy. 2018 Aug;107(3):202-208. doi: 10.1055/s-0038-1654709. Epub 2018 Jun 5. Homeopathy. 2018. PMID: 29871025 Clinical Trial.
-
[Use of antidepressant drugs in schizophrenic patients with depression].Encephale. 2006 Mar-Apr;32(2 Pt 1):263-9. doi: 10.1016/s0013-7006(06)76153-x. Encephale. 2006. PMID: 16910628 Review. French.
-
Novel Augmentation Strategies in Major Depression.Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review.
Cited by
-
[Homeopathy for psychiatric patients-for and against].Nervenarzt. 2018 Sep;89(9):1014-1019. doi: 10.1007/s00115-018-0540-2. Nervenarzt. 2018. PMID: 29858643 Review. German.
-
Correction: Individualized Homeopathic Treatment and Fluoxetine for Moderate to Severe Depression in Peri- and Postmenopausal Women (HOMDEP-MENOP Study): A Randomized, Double-Dummy, Double-Blind, Placebo-Controlled Trial.PLoS One. 2015 May 4;10(5):e0127719. doi: 10.1371/journal.pone.0127719. eCollection 2015. PLoS One. 2015. PMID: 25938465 Free PMC article. No abstract available.
-
Homeopathy Use in the United States and Implications for Public Health: A Review.Homeopathy. 2018 Feb;107(1):3-9. doi: 10.1055/s-0037-1609016. Epub 2017 Dec 22. Homeopathy. 2018. PMID: 29528473 Free PMC article. Review.
-
Placebo Effect in the Treatment of Depression and Anxiety.Front Psychiatry. 2019 Jun 13;10:407. doi: 10.3389/fpsyt.2019.00407. eCollection 2019. Front Psychiatry. 2019. PMID: 31249537 Free PMC article. Review.
-
Add-On Complementary Medicine in Cancer Care: Evidence in Literature and Experiences of Integration.Medicines (Basel). 2017 Jan 24;4(1):5. doi: 10.3390/medicines4010005. Medicines (Basel). 2017. PMID: 28930222 Free PMC article. Review.
References
-
- Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003; 74 (1): 5–13. - PubMed
-
- Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: The National Comorbidity Survey. Am J Psychiatry. 1994; 151 (7): 979–986. - PubMed
-
- Wise PM, Krajnak KM, Kashon ML. Menopause: the aging of multiple pacemakers. Science. 1996; 273: 67–70. - PubMed
-
- Shifren JL, Schiff I. The aging ovary. J Womens Health Gend Based Med 9 (suppl 1). 2000; S3–S7. - PubMed
-
- World Health Organization. Research on the Menopause in the 1990s Report of a WHO Scientific Group. WHO Technical Report Series; 1996. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

