Codon optimality is a major determinant of mRNA stability
- PMID: 25768907
- PMCID: PMC4359748
- DOI: 10.1016/j.cell.2015.02.029
Codon optimality is a major determinant of mRNA stability
Abstract
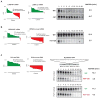
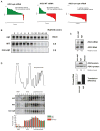
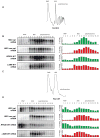
mRNA degradation represents a critical regulated step in gene expression. Although the major pathways in turnover have been identified, accounting for disparate half-lives has been elusive. We show that codon optimality is one feature that contributes greatly to mRNA stability. Genome-wide RNA decay analysis revealed that stable mRNAs are enriched in codons designated optimal, whereas unstable mRNAs contain predominately non-optimal codons. Substitution of optimal codons with synonymous, non-optimal codons results in dramatic mRNA destabilization, whereas the converse substitution significantly increases stability. Further, we demonstrate that codon optimality impacts ribosome translocation, connecting the processes of translation elongation and decay through codon optimality. Finally, we show that optimal codon content accounts for the similar stabilities observed in mRNAs encoding proteins with coordinated physiological function. This work demonstrates that codon optimization exists as a mechanism to finely tune levels of mRNAs and, ultimately, proteins.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




References
-
- Coller J, Parker R. Eukaryotic mRNA decapping. Annu Rev Biochem. 2004;73:861–890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

