Disulfiram (DSF) acts as a copper ionophore to induce copper-dependent oxidative stress and mediate anti-tumor efficacy in inflammatory breast cancer
- PMID: 25769405
- PMCID: PMC4493866
- DOI: 10.1016/j.molonc.2015.02.007
Disulfiram (DSF) acts as a copper ionophore to induce copper-dependent oxidative stress and mediate anti-tumor efficacy in inflammatory breast cancer
Abstract
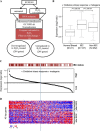
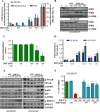
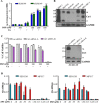
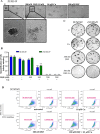
Cancer cells often have increased levels of reactive oxygen species (ROS); however, acquisition of redox adaptive mechanisms allows for evasion of ROS-mediated death. Inflammatory breast cancer (IBC) is a distinct, advanced BC subtype characterized by high rates of residual disease and recurrence despite advances in multimodality treatment. Using a cellular model of IBC, we identified an oxidative stress response (OSR) signature in surviving IBC cells after administration of an acute dose of an ROS inducer. Metagene analysis of patient samples revealed significantly higher OSR scores in IBC tumor samples compared to normal or non-IBC tissues, which may contribute to the poor response of IBC tumors to common treatment strategies, which often rely heavily on ROS induction. To combat this adaptation, we utilized a potent redox modulator, the FDA-approved small molecule Disulfiram (DSF), alone and in combination with copper. DSF forms a complex with copper (DSF-Cu) increasing intracellular copper concentration both in vitro and in vivo, bypassing the need for membrane transporters. DSF-Cu antagonized NFκB signaling, aldehyde dehydrogenase activity and antioxidant levels, inducing oxidative stress-mediated apoptosis in multiple IBC cellular models. In vivo, DSF-Cu significantly inhibited tumor growth without significant toxicity, causing apoptosis only in tumor cells. These results indicate that IBC tumors are highly redox adapted, which may render them resistant to ROS-inducing therapies. DSF, through redox modulation, may be a useful approach to enhance chemo- and/or radio-sensitivity for advanced BC subtypes where therapeutic resistance is an impediment to durable responses to current standard of care.
Keywords: ALDH; Antioxidant; Apoptosis; NFκB; SUM149; XIAP.
Copyright © 2015 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Abramoff, M.D. , Magalhaes, P.J. , Ram, S.J. , 2004. Image processing with ImageJ. Biophotonics Int. 11, 36–42.
-
- Aird, K.M. , Ding, X. , Baras, A. , Wei, J. , Morse, M.A. , Clay, T. , Lyerly, H.K. , Devi, G.R. , 2008. Trastuzumab signaling in ErbB2-overexpressing inflammatory breast cancer correlates with X-linked inhibitor of apoptosis protein expression. Mol. Cancer Ther. 7, 38–47. - PubMed
-
- Allensworth, J.L. , Aird, K.M. , Aldrich, A.J. , Batinic-Haberle, I. , Devi, G.R. , 2012. XIAP inhibition and generation of reactive oxygen species enhances TRAIL sensitivity in inflammatory breast cancer cells. Mol. Cancer Ther. 11, 1518–1527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

