Case-based reasoning using electronic health records efficiently identifies eligible patients for clinical trials
- PMID: 25769682
- PMCID: PMC4428438
- DOI: 10.1093/jamia/ocu050
Case-based reasoning using electronic health records efficiently identifies eligible patients for clinical trials
Abstract
Objective: To develop a cost-effective, case-based reasoning framework for clinical research eligibility screening by only reusing the electronic health records (EHRs) of minimal enrolled participants to represent the target patient for each trial under consideration.
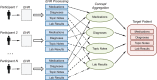
Materials and methods: The EHR data--specifically diagnosis, medications, laboratory results, and clinical notes--of known clinical trial participants were aggregated to profile the "target patient" for a trial, which was used to discover new eligible patients for that trial. The EHR data of unseen patients were matched to this "target patient" to determine their relevance to the trial; the higher the relevance, the more likely the patient was eligible. Relevance scores were a weighted linear combination of cosine similarities computed over individual EHR data types. For evaluation, we identified 262 participants of 13 diversified clinical trials conducted at Columbia University as our gold standard. We ran a 2-fold cross validation with half of the participants used for training and the other half used for testing along with other 30 000 patients selected at random from our clinical database. We performed binary classification and ranking experiments.
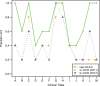
Results: The overall area under the ROC curve for classification was 0.95, enabling the highlight of eligible patients with good precision. Ranking showed satisfactory results especially at the top of the recommended list, with each trial having at least one eligible patient in the top five positions.
Conclusions: This relevance-based method can potentially be used to identify eligible patients for clinical trials by processing patient EHR data alone without parsing free-text eligibility criteria, and shows promise of efficient "case-based reasoning" modeled only on minimal trial participants.
Keywords: artificial intelligence; clinical trials; electronic health records; information storage and retrieval.
© The Author 2015. Published by Oxford University Press on behalf of the American Medical Informatics Association.
Figures


References
-
- Hersh WR. Adding value to the electronic health record through secondary use of data for quality assurance, research, and surveillance. Am J Manag Care. 2007;13(6):277–278. - PubMed
-
- Jensen PB, Jensen LJ, Brunak S. Mining electronic health records: towards better research applications and clinical care. Nat Rev Genet. 2012;13(6):395–405. - PubMed
-
- Sun J, Wang F, Hu J, Edabollahi S. Supervised patient similarity measure of heterogeneous patient records. ACM SIGKDD Explorations Newsletter. 2012;14(1):16–24.
-
- Wu J, Roy J, Stewart WF. Prediction modeling using EHR data: challenges, strategies, and a comparison of machine learning approaches. Med Care. 2010;48(6 Suppl):S106–S113. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

