Paracrine WNT5A Signaling Inhibits Expansion of Tumor-Initiating Cells
- PMID: 25769722
- PMCID: PMC4433621
- DOI: 10.1158/0008-5472.CAN-14-2761
Paracrine WNT5A Signaling Inhibits Expansion of Tumor-Initiating Cells
Abstract
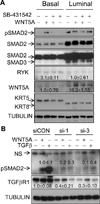
It is not well understood how paracrine communication between basal and luminal cell populations in the mammary gland affects tumorigenesis. During ErbB2-induced mammary tumorigenesis, enriched mammary stem cells that represent a subpopulation of basal cells exhibit enhanced tumorigenic capacity compared with the corresponding luminal progenitors. Transcript profiling of tumors derived from basal and luminal tumor-initiating cells (TIC) revealed preferential loss of the noncanonical Wnt ligand WNT5A in basal TIC-derived tumors. Heterozygous loss of WNT5A was correlated with shorter survival of breast cancer patients. In a mouse model of ErbB2-induced breast cancer, Wnt5a heterozygosity promoted tumor multiplicity and pulmonary metastasis. As a TGFβ substrate, luminal cell-produced WNT5A induced a feed-forward loop to activate SMAD2 in a RYK and TGFβR1-dependent manner to limit the expansion of basal TIC in a paracrine fashion, a potential explanation for the suppressive effect of WNT5A in mammary tumorigenesis. Our results identify the WNT5A/RYK module as a spatial regulator of the TGFβ-SMAD signaling pathway in the context of mammary gland development and carcinogenesis, offering a new perspective on tumor suppression provided by basal-luminal cross-talk in normal mammary tissue.
©2015 American Association for Cancer Research.
Conflict of interest statement
All authors possess no conflicts of interest.
Figures





References
-
- Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411(6835):349–354. - PubMed
-
- Wansleeben C, Meijlink F. The planar cell polarity pathway in vertebrate development. Developmental Dynamics. 2011;240(3):616–626. - PubMed
-
- Slusarski DC, Yang-Snyder J, Busa WB, Moon RT. Modulation of Embryonic Intracellular Ca2+Signaling byWnt-5A. Developmental Biology. 1997;182(1):114–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

