Defense-in-depth by mucosally administered anti-HIV dimeric IgA2 and systemic IgG1 mAbs: complete protection of rhesus monkeys from mucosal SHIV challenge
- PMID: 25769884
- PMCID: PMC4411954
- DOI: 10.1016/j.vaccine.2015.02.020
Defense-in-depth by mucosally administered anti-HIV dimeric IgA2 and systemic IgG1 mAbs: complete protection of rhesus monkeys from mucosal SHIV challenge
Abstract
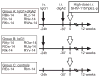
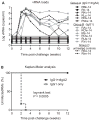
Although IgA is the most abundantly produced immunoglobulin in humans, its role in preventing HIV-1 acquisition, which occurs mostly via mucosal routes, remains unclear. In our passive mucosal immunizations of rhesus macaques (RMs), the anti-HIV-1 neutralizing monoclonal antibody (nmAb) HGN194, given either as dimeric IgA1 (dIgA1) or dIgA2 intrarectally (i.r.), protected 83% or 17% of the RMs against i.r. simian-human immunodeficiency virus (SHIV) challenge, respectively. Data from the RV144 trial implied that vaccine-induced plasma IgA counteracted the protective effector mechanisms of IgG1 with the same epitope specificity. We thus hypothesized that mucosal dIgA2 might diminish the protection provided by IgG1 mAbs targeting the same epitope. To test our hypothesis, we administered HGN194 IgG1 intravenously (i.v.) either alone or combined with i.r. HGN194 dIgA2. We enrolled SHIV-exposed, persistently aviremic RMs protected by previously administered nmAbs; RM anti-human IgG responses were undetectable. However, low-level SIV Gag-specific proliferative T-cell responses were found. These animals resemble HIV-exposed, uninfected humans, in which local and systemic cellular immune responses have been observed. HGN194 IgG1 and dIgA2 used alone and the combination of the two neutralized the challenge virus equally well in vitro. All RMs given only i.v. HGN194 IgG1 became infected. In contrast, all RMs given HGN194 IgG1+dIgA2 were completely protected against high-dose i.r. SHIV-1157ipEL-p challenge. These data imply that combining suboptimal defenses at the mucosal and systemic levels can completely prevent virus acquisition. Consequently, active vaccination should focus on defense-in-depth, a strategy that seeks to build up defensive fall-back positions well behind the fortified frontline.
Keywords: (6 max); Complete protection; IgA; IgG; Passive immunization; Rhesus monkey; SHIV-C mucosal challenge.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


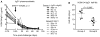


Similar articles
-
Cooperation Between Systemic IgG1 and Mucosal Dimeric IgA2 Monoclonal Anti-HIV Env Antibodies: Passive Immunization Protects Indian Rhesus Macaques Against Mucosal SHIV Challenges.Front Immunol. 2021 Aug 3;12:705592. doi: 10.3389/fimmu.2021.705592. eCollection 2021. Front Immunol. 2021. PMID: 34413855 Free PMC article.
-
Anti-HIV IgA isotypes: differential virion capture and inhibition of transcytosis are linked to prevention of mucosal R5 SHIV transmission.AIDS. 2013 Jun 1;27(9):F13-20. doi: 10.1097/QAD.0b013e328360eac6. AIDS. 2013. PMID: 23775002 Free PMC article.
-
Protective Efficacy of Broadly Neutralizing Antibodies with Incomplete Neutralization Activity against Simian-Human Immunodeficiency Virus in Rhesus Monkeys.J Virol. 2017 Sep 27;91(20):e01187-17. doi: 10.1128/JVI.01187-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28768869 Free PMC article.
-
Are anti-HIV IgAs good guys or bad guys?Retrovirology. 2014 Dec 14;11:109. doi: 10.1186/s12977-014-0109-5. Retrovirology. 2014. PMID: 25499540 Free PMC article. Review.
-
Antibody-mediated immune exclusion of HIV.Curr Opin HIV AIDS. 2017 May;12(3):222-228. doi: 10.1097/COH.0000000000000369. Curr Opin HIV AIDS. 2017. PMID: 28422786 Free PMC article. Review.
Cited by
-
Predicting HIV-1 transmission and antibody neutralization efficacy in vivo from stoichiometric parameters.PLoS Pathog. 2017 May 4;13(5):e1006313. doi: 10.1371/journal.ppat.1006313. eCollection 2017 May. PLoS Pathog. 2017. PMID: 28472201 Free PMC article.
-
Mucosal IgA Responses: Damaged in Established HIV Infection-Yet, Effective Weapon against HIV Transmission.Front Immunol. 2017 Nov 15;8:1581. doi: 10.3389/fimmu.2017.01581. eCollection 2017. Front Immunol. 2017. PMID: 29176985 Free PMC article. Review.
-
Mucosal Vaccine Approaches for Prevention of HIV and SIV Transmission.Curr Immunol Rev. 2019;15(1):102-122. doi: 10.2174/1573395514666180605092054. Curr Immunol Rev. 2019. PMID: 31452652 Free PMC article.
-
Optimized Mucosal Modified Vaccinia Virus Ankara Prime/Soluble gp120 Boost HIV Vaccination Regimen Induces Antibody Responses Similar to Those of an Intramuscular Regimen.J Virol. 2019 Jun 28;93(14):e00475-19. doi: 10.1128/JVI.00475-19. Print 2019 Jul 15. J Virol. 2019. PMID: 31068425 Free PMC article.
-
IgA Targeting Human Immunodeficiency Virus-1 Envelope gp41 Triggers Antibody-Dependent Cellular Cytotoxicity Cross-Clade and Cooperates with gp41-Specific IgG to Increase Cell Lysis.Front Immunol. 2018 Mar 29;9:244. doi: 10.3389/fimmu.2018.00244. eCollection 2018. Front Immunol. 2018. PMID: 29651286 Free PMC article.
References
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. - PubMed
-
- Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

