Community participation in biofilm matrix assembly and function
- PMID: 25770218
- PMCID: PMC4386410
- DOI: 10.1073/pnas.1421437112
Community participation in biofilm matrix assembly and function
Abstract
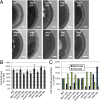
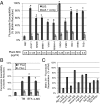
Biofilms of the fungus Candida albicans produce extracellular matrix that confers such properties as adherence and drug resistance. Our prior studies indicate that the matrix is complex, with major polysaccharide constituents being α-mannan, β-1,6 glucan, and β-1,3 glucan. Here we implement genetic, biochemical, and pharmacological approaches to unravel the contributions of these three constituents to matrix structure and function. Interference with synthesis or export of any one polysaccharide constituent altered matrix concentrations of each of the other polysaccharides. Each of these was also required for matrix function, as assessed by assays for sequestration of the antifungal drug fluconazole. These results indicate that matrix biogenesis entails coordinated delivery of the individual matrix polysaccharides. To understand whether coordination occurs at the cellular level or the community level, we asked whether matrix-defective mutant strains could be coaxed to produce functional matrix through biofilm coculture. We observed that mixed biofilms inoculated with mutants containing a disruption in each polysaccharide pathway had restored mature matrix structure, composition, and biofilm drug resistance. Our results argue that functional matrix biogenesis is coordinated extracellularly and thus reflects the cooperative actions of the biofilm community.
Keywords: Candida; biofilm; matrix; polysaccharide; resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284(5418):1318–1322. - PubMed
-
- Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol. 2009;11(7):1034–1043. - PubMed
-
- National Institutes of Health 1999. SBIR/STTR study and control of microbial biofilms. Available at grants.nih.gov/grants/guide/pa-files/PA-99-084.html. Accessed May 21, 2014.
-
- Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9(1):34–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

