A systematic review of controlled trials of lower-protein or energy-containing infant formulas for use by healthy full-term infants
- PMID: 25770256
- PMCID: PMC4352176
- DOI: 10.3945/an.114.006379
A systematic review of controlled trials of lower-protein or energy-containing infant formulas for use by healthy full-term infants
Abstract
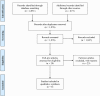
Infant formulas have historically been developed based on providing macronutrients at intake concentrations approximately matching the composition of human milk. In most countries, targets of 1.4-1.5 g of protein/dL and 20 kcal/oz (67-68 kcal/dL) have been set as the protein and energy concentrations for formulas during the first year of life, although this may be an overestimation of these contents. Recent introduction of lower-protein and -energy formulas in full-term infants led us to systematically review the literature for its effects on growth. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines, our inclusion criteria were studies that enrolled healthy full-term infants and evaluated lower-protein or lower-energy formula, reported anthropometric outcomes including weight and length, and followed infants for at least 6 mo. Six studies were eligible for inclusion. These studies varied in the content of nutrients provided in the intervention and control groups, by additional dietary components in the study groups, and the timing and length of the intervention, which limit their usefulness for interpreting newly introduced lower-protein and -energy formulas in the United States. These studies suggest adequate growth during infancy and early childhood with infant formulas with concentrations of protein and energy slightly below historical standards in the United States. Further long-term research is needed to assess the impact of the use of lower-protein and/or lower-energy products, especially for nutritionally at-risk populations such as preterm infants and infants who are born small for gestational age.
Keywords: child growth; development; infant formula; lactation; obesity prevention; protein requirements.
© 2015 American Society for Nutrition.
Conflict of interest statement
Author disclosures: SA Abrams receives research funding for a project unrelated to the topic of this report from Mead Johnson Nutrition. KM Hawthorne participates as a speaker for Abbott Nutrition and Mead Johnson Nutrition. M Pammi, no conflicts of interest.
Figures
References
-
- Fomon SJ, Ziegler EE, Nelson SE, Frantz JA. What is the safe protein-energy ratio for infant formulas? Am J Clin Nutr 1995;62:358–63. - PubMed
-
- Food and Drug Administration [Internet]. Regulations and information on the manufacture and distribution of infant formula, 2002 [cited 2014 Jul 29]. Available from: www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformati....
-
- Assessment of nutrient requirements for infant formulas. J Nutr 1998;128 Suppl:i-iv, 2059S–2293S. - PubMed
-
- Codex Alimentarius International Food Standards. Standard for infant formula and formulas for special medical purposes intended for infants [cited 2014 Sep 11]. Available from: www.codexalimentarius.org.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical