Low-dose TNF augments fracture healing in normal and osteoporotic bone by up-regulating the innate immune response
- PMID: 25770819
- PMCID: PMC4492816
- DOI: 10.15252/emmm.201404487
Low-dose TNF augments fracture healing in normal and osteoporotic bone by up-regulating the innate immune response
Abstract
The mechanism by which trauma initiates healing remains unclear. Precise understanding of these events may define interventions for accelerating healing that could be translated to the clinical arena. We previously reported that addition of low-dose recombinant human TNF (rhTNF) at the fracture site augmented fracture repair in a murine tibial fracture model. Here, we show that local rhTNF treatment is only effective when administered within 24 h of injury, when neutrophils are the major inflammatory cell infiltrate. Systemic administration of anti-TNF impaired fracture healing. Addition of rhTNF enhanced neutrophil recruitment and promoted recruitment of monocytes through CCL2 production. Conversely, depletion of neutrophils or inhibition of the chemokine receptor CCR2 resulted in significantly impaired fracture healing. Fragility, or osteoporotic, fractures represent a major medical problem as they are associated with permanent disability and premature death. Using a murine model of fragility fractures, we found that local rhTNF treatment improved fracture healing during the early phase of repair. If translated clinically, this promotion of fracture healing would reduce the morbidity and mortality associated with delayed patient mobilization.
Keywords: CCL2; TNF; bone; fracture; inflammation.
© 2015 The Authors. Published under the terms of the CC BY 4.0 license.
Figures

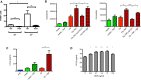
Addition of rhTNF at the fracture site on days 0 and 1 led to augmented healing, indicated by increased % callus mineralization, at day 28 after operation in a dose-dependent manner. Data are presented as mean ± SEM. Treatment with 1 ng TNF versus PBS control, ****P < 0.0001 by one-way ANOVA with Dunnett's multiple comparisons test.
Addition of rhTNF at the fracture site must be given within the first 24 h to augment healing, indicated by % callus mineralization. Data are presented as mean ± SEM. 1 ng TNF versus PBS control treatment on days 0 and 1, ***P = 0.0009 by one-way ANOVA with Dunnett's multiple comparisons test.
Treatment with systemic anti-TNF or local rmIL-10 led to impaired fracture repair at day 28, indicated by % callus mineralization. Data are presented as mean ± SEM. Treatment with PBS control versus anti-TNF, *P = 0.037, PBS control versus rmIL-10, *P = 0.037, by unpaired two-sided t-tests.
Representative micro-CT images at the fracture site showing (from left to right): lateral view of tibia, cross-section, and cross and longitudinal sections with color overlay and 3D reconstruction. In the color overlays, the shade of blue corresponds to percentage mineralization: light blue denotes soft immature callus, whereas dark blue denotes hard mineralized callus. Scale bar, 2 mm.
Representative ISH image (light field) showing TNF expression at the murine fracture site at 24 h after fracture. Scale bar, 250 μm. Red box indicates region of interest.
High-power micrographs of region of interest from (E): at 24 h after fracture, mTNF expression (white signal on dark field, above) co-localized with polymorphonuclear cells found on the adjacent H&E section (below). Scale bar, 25 μm. Neutrophils were identified by their polymorphonuclear morphology as well as positive dark brown staining with anti-neutrophil elastase.
High-power micrographs: at 7 days, TNF expression (white signal on dark field, above) co-localized with F4/80-positive cells (stained dark brown) extravasating from a blood vessel on the adjacent H&E section (below). Scale bar, 25 μm.

Neutrophils were mobilized into the murine systemic circulation within 30 min of injury in our murine fracture model. Compared to control and number of blood neutrophils at 3 h ***P = 0.0006 and at 6 h *P = 0.042, by one-way ANOVA with Dunnett correction.
Depletion of neutrophils using anti-Ly6G. Intravenous anti-Ly6G treatment led to depletion of neutrophils in the systemic circulation (left) as well the local peri-fracture soft tissues (right). Left: counts of neutrophils in the blood harvested by cardiac puncture at 3 h post-injury. Data are presented as mean ± SEM (**P = 0.0074 using unpaired one-tailed t-test). Right: counts of positively stained infiltrative neutrophils in the adjacent muscle to the fracture site comparing neutrophil depletion with anti-Ly6G antibody versus IgG control at day 1 and day 7 post-fracture. At day 1, number of neutrophils in control group as detected by Ly6G and NE in control versus treatment: ****P = 0.0001 for both Ly6G and NE, by unpaired two-tailed t-test. At day 7, number of neutrophils in control group as detected by Ly6G and NE in control versus treatment groups: ****P = 0.0001 for Ly6G and *P = 0.020 for NE, by unpaired two-tailed t-test. Number of neutrophils at day 1 versus day 7 in control groups, *P = 0.037 for Ly6G and ***P = 0.0006 for NE, by unpaired two-tailed t-test.
Representative sections showing local infiltration of neutrophils in the adjacent muscle stained using anti-Ly6G and anti-neutrophil elastase primary antibodies at day 1 post-fracture following treatment with anti-Ly6G antibody or IgG control. Scale bar, 100 μm.
Depletion of neutrophils using anti-Ly6G led to impaired fracture healing. Representative section stained with Masson's trichrome at day 14 comparing fracture healing in mouse treated with isotype control (top) versus anti-Ly6G (bottom). Black and white images in the right column are identical to the color images in the left column; they have been labeled to clearly demonstrate the anatomical structures. Control section shows advanced mineralized callus, while treatment section shows a large immature unmineralized callus. Muscle fibers: red; collagen, bone, and mineralized callus: green. Scale bar, 1 mm.
Anti-Ly6G treatment led to impaired fracture healing as shown by the reduced % callus mineralization at day 28 after surgery and representative micro-CT images. Anti-Ly6G treatment led to delayed mineralization and remodeling of the fracture callus compared to control. Data are presented as mean ± SEM. ***P = 0.0006 by unpaired two-sided t-test. Scale Bar, 2 mm.
Anti-Ly6G treatment depletes local monocytes/macrophages at the fracture site. Counts of positively stained infiltrative F4/80+ cells in the adjacent muscle to the fracture site following treatment with control or anti-Ly6G at day 1 and day 7. Data are presented as mean ± SEM. **P = 0.005 at day 1 and **P = 0.002 at day 7, and *P = 0.013 for control at day 1 versus control at day 7, by unpaired two-sided t-test.
Addition of 1 ng rhTNF to fracture supernatant in the air pouch model led to increased numbers of neutrophils (Ly6G+, CD11b+ cells) and monocytes/macrophages (Ly6G−, CD11b+, CD115+ cells) in the cellular infiltrate. These effects were abrogated by the addition of anti-CCL2. Neutrophils: FS versus FS + TNF *P = 0.015, FS + TNF versus FS + TNF + anti-CCL2 *P = 0.028, and FS + TNF + anti-CCL2 versus FS + TNF + IgG *P = 0.042 by one-way ANOVA followed by Bonferroni's multiple comparison test. Monocytes/macrophages: FS versus FS + TNF **P = 0.0063, FS + TNF versus FS + TNF + anti-CCL2 ****P < 0.0001, and FS + TNF + anti-CCL2 v FS + TNF + IgG *P = 0.015 by one-way ANOVA followed by Bonferroni's multiple comparisons test.
Addition of 1 ng rhTNF to fracture supernatant increased CCL2 protein levels in the air pouch. “Neat” indicates level of CCL2 in fracture supernatant before injection into air pouch. Data are presented as mean ± SEM. FS versus FS + TNF **P = 0.0020 by one-way ANOVA with Bonferroni's multiple comparisons test.
Addition of rhTNF to enriched bone marrow-derived murine neutrophils pre-exposed to fracture supernatant in vitro led to up-regulation of CCL2 production at 1 h. Data are presented as mean ± SEM. No TNF versus TNF 0.01 ng/ml *P = 0.012, TNF 0.1 ng/ml ***P < 0.0001, TNF 1.0 ng ***P = 0.0001, TNF 10 ng/ml ***P < 0.0001, and TNF 100 ng/ml *P = 0.023 by one-way ANOVA with Dunnett's multiple comparisons test.

Similar articles
-
TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells.Proc Natl Acad Sci U S A. 2011 Jan 25;108(4):1585-90. doi: 10.1073/pnas.1018501108. Epub 2011 Jan 5. Proc Natl Acad Sci U S A. 2011. PMID: 21209334 Free PMC article.
-
Fracture healing in osteoporotic bone.Injury. 2016 Jun;47 Suppl 2:S21-6. doi: 10.1016/S0020-1383(16)47004-X. Injury. 2016. PMID: 27338222 Review.
-
The crucial role of neutrophil granulocytes in bone fracture healing.Eur Cell Mater. 2016 Jul 25;32:152-62. doi: 10.22203/ecm.v032a10. Eur Cell Mater. 2016. PMID: 27452963
-
TNF-α is upregulated in T2DM patients with fracture and promotes the apoptosis of osteoblast cells in vitro in the presence of high glucose.Cytokine. 2016 Apr;80:35-42. doi: 10.1016/j.cyto.2016.01.011. Epub 2016 Mar 3. Cytokine. 2016. PMID: 26945994
-
Drugs for bone healing.Expert Opin Investig Drugs. 2012 Aug;21(8):1169-76. doi: 10.1517/13543784.2012.696610. Epub 2012 Jun 14. Expert Opin Investig Drugs. 2012. PMID: 22694479 Review.
Cited by
-
The impact of immune response on endochondral bone regeneration.NPJ Regen Med. 2018 Nov 29;3:22. doi: 10.1038/s41536-018-0060-5. eCollection 2018. NPJ Regen Med. 2018. PMID: 30510772 Free PMC article. Review.
-
Effects of immune cells on mesenchymal stem cells during fracture healing.World J Stem Cells. 2021 Nov 26;13(11):1667-1695. doi: 10.4252/wjsc.v13.i11.1667. World J Stem Cells. 2021. PMID: 34909117 Free PMC article. Review.
-
The Role of Osteocytes in Inflammatory Bone Loss.Front Endocrinol (Lausanne). 2019 May 14;10:285. doi: 10.3389/fendo.2019.00285. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31139147 Free PMC article. Review.
-
Five Days Granulocyte Colony-Stimulating Factor Treatment Increases Bone Formation and Reduces Gap Size of a Rat Segmental Bone Defect: A Pilot Study.Front Bioeng Biotechnol. 2018 Feb 12;6:5. doi: 10.3389/fbioe.2018.00005. eCollection 2018. Front Bioeng Biotechnol. 2018. PMID: 29484293 Free PMC article.
-
Modulating macrophage activities to promote endogenous bone regeneration: Biological mechanisms and engineering approaches.Bioact Mater. 2020 Aug 22;6(1):244-261. doi: 10.1016/j.bioactmat.2020.08.012. eCollection 2021 Jan. Bioact Mater. 2020. PMID: 32913932 Free PMC article. Review.
References
-
- Alexander KA, Chang MK, Maylin ER, Kohler T, Muller R, Wu AC, Van Rooijen N, Sweet MJ, Hume DA, Raggatt LJ, et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res. 2011;26:1517–1532. - PubMed
-
- Altman RD, Latta LL, Keer R, Renfree K, Hornicek FJ, Banovac K. Effect of nonsteroidal antiinflammatory drugs on fracture healing: a laboratory study in rats. J Orthop Trauma. 1995;9:392–400. - PubMed
-
- Arefieva TI, Sokolov VO, Pylaeva EA, Kukhtina NB, Potekhina AV, Ruleva NY, Sidorova MV, Bespalova Z, Azmuko AA, Krasnikova TL. Peptide fragment 29–40 of amino acid sequence of monocyte chemoattractant protein-1 (MCP-1) stimulates monocyte migration in vivo and facilitates wound healing. Dokl Biol Sci. 2012;446:327–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

