Mussel adhesive protein provides cohesive matrix for collagen type-1α
- PMID: 25770997
- PMCID: PMC4361793
- DOI: 10.1016/j.biomaterials.2015.01.033
Mussel adhesive protein provides cohesive matrix for collagen type-1α
Abstract
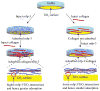
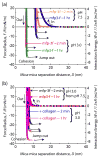
Understanding the interactions between collagen and adhesive mussel foot proteins (mfps) can lead to improved medical and dental adhesives, particularly for collagen-rich tissues. Here we investigated interactions between collagen type-1, the most abundant load-bearing animal protein, and mussel foot protein-3 (mfp-3) using a quartz crystal microbalance and surface forces apparatus (SFA). Both hydrophilic and hydrophobic variants of mfp-3 were exploited to probe the nature of the interaction between the protein and collagen. Our chief findings are: 1) mfp-3 is an effective chaperone for tropocollagen adsorption to TiO2 and mica surfaces; 2) at pH 3, collagen addition between two mfp-3 films (Wc = 5.4 ± 0.2 mJ/m(2)) increased their cohesion by nearly 35%; 3) oxidation of Dopa in mfp-3 by periodate did not abolish the adhesion between collagen and mfp-3 films, and 4) collagen bridging between both hydrophilic and hydrophobic mfp-3 variant films is equally robust, suggesting that hydrophobic interactions play a minor role. Extensive H-bonding, π-cation and electrostatic interactions are more plausible to explain the reversible bridging of mfp-3 films by collagen.
Keywords: Collagen type-1; Mfp-3; Mussel foot proteins; Mylitus californiaus.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
Adhesion of mussel foot proteins to different substrate surfaces.J R Soc Interface. 2013 Feb;10(79):20120759. doi: 10.1098/rsif.2012.0759. J R Soc Interface. 2013. PMID: 23173195 Free PMC article.
-
Adhesion mechanisms of the mussel foot proteins mfp-1 and mfp-3.Proc Natl Acad Sci U S A. 2007 Mar 6;104(10):3782-6. doi: 10.1073/pnas.0607852104. Epub 2007 Feb 28. Proc Natl Acad Sci U S A. 2007. PMID: 17360430 Free PMC article.
-
Adaptive hydrophobic and hydrophilic interactions of mussel foot proteins with organic thin films.Proc Natl Acad Sci U S A. 2013 Sep 24;110(39):15680-5. doi: 10.1073/pnas.1315015110. Epub 2013 Sep 6. Proc Natl Acad Sci U S A. 2013. PMID: 24014592 Free PMC article.
-
Mussel adhesion - essential footwork.J Exp Biol. 2017 Feb 15;220(Pt 4):517-530. doi: 10.1242/jeb.134056. J Exp Biol. 2017. PMID: 28202646 Free PMC article. Review.
-
Cation-π Interactions and Their Contribution to Mussel Underwater Adhesion Studied Using a Surface Forces Apparatus: A Mini-Review.Langmuir. 2019 Dec 3;35(48):16002-16012. doi: 10.1021/acs.langmuir.9b01976. Epub 2019 Aug 26. Langmuir. 2019. PMID: 31423790 Review.
Cited by
-
Mussel-inspired biomaterials: From chemistry to clinic.Bioeng Transl Med. 2022 Aug 11;7(3):e10385. doi: 10.1002/btm2.10385. eCollection 2022 Sep. Bioeng Transl Med. 2022. PMID: 36176595 Free PMC article. Review.
-
High-performance mussel-inspired adhesives of reduced complexity.Nat Commun. 2015 Oct 19;6:8663. doi: 10.1038/ncomms9663. Nat Commun. 2015. PMID: 26478273 Free PMC article.
-
Biomimetic characteristics of mussel adhesive protein-loaded collagen membrane in guided bone regeneration of rabbit calvarial defects.J Periodontal Implant Sci. 2018 Oct 24;48(5):305-316. doi: 10.5051/jpis.2018.48.5.305. eCollection 2018 Oct. J Periodontal Implant Sci. 2018. PMID: 30405938 Free PMC article.
-
Peptide Length and Dopa Determine Iron-Mediated Cohesion of Mussel Foot Proteins.Adv Funct Mater. 2015 Sep 23;25(36):5840-5847. doi: 10.1002/adfm.201502256. Epub 2015 Aug 17. Adv Funct Mater. 2015. PMID: 28670243 Free PMC article.
-
Advances in Soft and Dry Electrodes for Wearable Health Monitoring Devices.Micromachines (Basel). 2022 Apr 16;13(4):629. doi: 10.3390/mi13040629. Micromachines (Basel). 2022. PMID: 35457934 Free PMC article. Review.
References
-
- Rojkind M, Giambrone MA, Biempica L. Collagen Types in Normal and Cirrhotic Liver. Gastroenterology. 1979;76:710–9. - PubMed
-
- Riechert K, Labs K, Lindenhayn K, Sinha P. Semiquantitative analysis of types I and III collagen from tendons and ligaments in a rabbit model. J Orthop Sci. 2001;6:68–74. - PubMed
-
- Heino J. The collagen family members as cell adhesion proteins. Bioessays. 2007;29:1001–10. - PubMed
-
- Basser PJ, Schneiderman R, Bank RA, Wachtel E, Maroudas A. Mechanical properties of the collagen network in human articular cartilage as measured by osmotic stress technique. Arch Biochem Biophys. 1998;351:207–19. - PubMed
-
- Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-Healing in a Bone Tunnel - a Biomechanical and Histological Study in the Dog. J Bone Joint Surg Am. 1993;75A:1795–803. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

