miRNAs in atherosclerotic plaque initiation, progression, and rupture
- PMID: 25771097
- PMCID: PMC4424146
- DOI: 10.1016/j.molmed.2015.02.003
miRNAs in atherosclerotic plaque initiation, progression, and rupture
Abstract
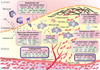
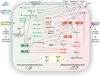
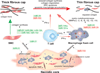
Atherosclerosis is a chronic immune-inflammatory disorder that integrates multiple cell types and a diverse set of inflammatory mediators. miRNAs are emerging as important post-transcriptional regulators of gene expression in most, if not all, vertebrate cells, and constitute central players in many physiological and pathological processes. Rapidly accumulating experimental studies reveal their key role in cellular and molecular processes related to the development of atherosclerosis. We review current evidence for the involvement of miRNAs in early atherosclerotic lesion formation and in plaque rupture and erosion. We conclude with a perspective on the clinical relevance, therapeutic opportunities, and future challenges of miRNA biology in understanding the pathogenesis of this complex disease.
Keywords: atherosclerosis; inflammation; miRNAs; shear stress; vulnerable plaque.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
References
-
- Zhu N, et al. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis. 2011;215:286–293. - PubMed
-
- Sun HX, et al. Essential role of microRNA-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension. 2012;60:1407–1414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

