An Investigation on the Effect of Polyethylene Oxide Concentration and Particle Size in Modulating Theophylline Release from Tablet Matrices
- PMID: 25771738
- PMCID: PMC4666262
- DOI: 10.1208/s12249-015-0295-z
An Investigation on the Effect of Polyethylene Oxide Concentration and Particle Size in Modulating Theophylline Release from Tablet Matrices
Abstract
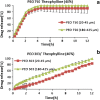
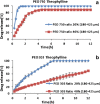
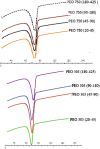
Polyethylene oxide has been researched extensively as an alternative polymer to hydroxypropyl methylcellulose (HPMC) in controlled drug delivery due to its desirable swelling properties and its availability in a number of different viscosity grades. Previous studies on HPMC have pointed out the importance of particle size on drug release, but as of yet, no studies have investigated the effect of particle size of polyethylene oxide (polyox) on drug release. The present study explored the relationship between polymer level and particle size to sustain the drug release. Tablets produced contained theophylline as their active ingredient and consisted of different polyethylene oxide particle size fractions (20-45, 45-90, 90-180 and 180-425 μm). It was shown that matrices containing smaller particle sizes of polyox produced harder tablets than when larger polyox particles were used. The release studies showed that matrices consisting of large polyox particles showed a faster release rate than matrices made from smaller particles. Molecular weight (MW) of the polymer was a key determining step in attaining sustained release, with the high MW of polyox resulting in a delayed release profile. The results showed that the effect of particle size on drug release was more detrimental when a low concentration of polyox was used. This indicates that care must be taken when low levels of polyox with different particle size fractions are used. More robust formulations could be obtained when the concentration of polyox is high. Differential scanning calorimetry (DSC) traces showed that particle size had no major effect on the thermal behaviour of polyox particles.
Keywords: DSC traces; particle size; polyox; sustained release; theophylline.
Figures



Similar articles
-
Evaluation of the drug solubility and rush ageing on drug release performance of various model drugs from the modified release polyethylene oxide matrix tablets.Drug Deliv Transl Res. 2017 Feb;7(1):111-124. doi: 10.1007/s13346-016-0344-5. Drug Deliv Transl Res. 2017. PMID: 27873080 Free PMC article.
-
Investigation of hypromellose particle size effects on drug release from sustained release hydrophilic matrix tablets.Drug Dev Ind Pharm. 2007 Sep;33(9):952-8. doi: 10.1080/03639040601134132. Drug Dev Ind Pharm. 2007. PMID: 17891581
-
Film-coated matrix mini-tablets for the extended release of a water-soluble drug.Drug Dev Ind Pharm. 2015 Apr;41(4):623-30. doi: 10.3109/03639045.2014.891128. Epub 2014 Feb 24. Drug Dev Ind Pharm. 2015. PMID: 24564797
-
Polyethylene oxide and its controlled release properties in hydrophilic matrix tablets for oral administration.Pharm Dev Technol. 2020 Dec;25(10):1169-1187. doi: 10.1080/10837450.2020.1808015. Epub 2020 Aug 20. Pharm Dev Technol. 2020. PMID: 32772604 Review.
-
Applications of poly(ethylene oxide) in controlled release tablet systems: a review.Drug Dev Ind Pharm. 2014 Jul;40(7):845-51. doi: 10.3109/03639045.2013.831438. Epub 2013 Sep 3. Drug Dev Ind Pharm. 2014. PMID: 24001212 Review.
References
-
- Haan PD, Lerk CF. Oral controlled release dosage forms: a review. Pharm World Sci. 1984;6:57–67. - PubMed
-
- Bailey FE, Koleske JV. Alkylene oxides and their polymer. New York: Marcel and Dekker; 1990.
-
- Bailey FE, Koleske JV. Poly(ethylene oxide) New York: Academic; 1976.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

