Lactose-free milk for infants with acute gastroenteritis in a developing country: study protocol for a randomized controlled trial
- PMID: 25771831
- PMCID: PMC4324790
- DOI: 10.1186/s13063-015-0565-9
Lactose-free milk for infants with acute gastroenteritis in a developing country: study protocol for a randomized controlled trial
Abstract
Background: Acute gastroenteritis is a major cause of pediatric morbidity and mortality, accounting for 15% of all childhood deaths worldwide. In developing countries, diarrheal diseases continue to be a major public health burden. Evidence from developed countries suggests that intake of lactose-free milk during diarrheal episodes may reduce the duration of the illness in pediatric inpatients. It is unknown whether lactose-free milk reduces the severity or duration of acute gastroenteritis in infants treated in outpatient settings in developing countries where diarrhea is more severe, and results in higher morbidities and mortalities. We hypothesize that lactose-free milk intake during acute gastroenteritis would significantly decrease the duration and severity of diarrhea in infants presenting to the Emergency Department (ED), as compared with lactose-containing milk.
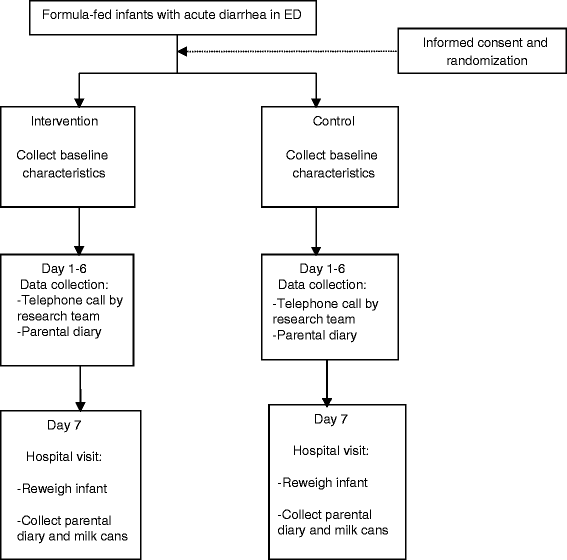
Methods/design: An open-label randomized clinical trial.
Study population: 40 infants with acute gastroenteritis, age between 2 and 12 months, presenting to the ED, will be randomized to control or intervention group.
Intervention: Lactose-free milk, whereas the control group will continue on regular infant formula for a total of 7 days. Infants will be followed up for 7 days.
Outcome measures: Diarrhea duration, weight loss, illness clinic visits, hospitalization rate, parental satisfaction, and time to symptom resolution.
Statistical analysis: Descriptive and regression analysis will be conducted under the intention-to-treat basis by using SPSS version 21.
Discussion: Acute gastroenteritis is a public health burden for developing countries, with a significant impact on infant morbidity and mortality. Provision of infant formula that may reduce the duration and severity of diarrhea can decrease this burden in countries with limited healthcare resources, like Lebanon. The findings from this study are anticipated to provide evidence-based dietary recommendations for ambulatory infants with acute diarrhea in developing countries.
Trial registration: ClinicalTrials.gov NCT02246010; September 2014.
Figures
Similar articles
-
Cow's milk-based formula, human milk, and soya feeds in acute infantile diarrhea: a therapeutic trial.J Pediatr Gastroenterol Nutr. 1990 Feb;10(2):193-8. doi: 10.1097/00005176-199002000-00009. J Pediatr Gastroenterol Nutr. 1990. PMID: 2406406 Clinical Trial.
-
Lactose-free formulae for infantile diarrhoea.Lancet. 1980 Jan 26;1(8161):207. doi: 10.1016/s0140-6736(80)90694-7. Lancet. 1980. PMID: 6101662 Clinical Trial. No abstract available.
-
The nutritional management of acute diarrhea in young infants: effect of carbohydrate ingested.J Pediatr Gastroenterol Nutr. 1994 Aug;19(2):170-4. doi: 10.1097/00005176-199408000-00005. J Pediatr Gastroenterol Nutr. 1994. PMID: 7815238 Clinical Trial.
-
Dietary management of acute diarrheal disease: contemporary scientific issues.J Nutr. 1994 Aug;124(8 Suppl):1455S-1460S. doi: 10.1093/jn/124.suppl_8.1455S. J Nutr. 1994. PMID: 8064403 Review.
-
Dietary modifications for infantile colic.Cochrane Database Syst Rev. 2018 Oct 10;10(10):CD011029. doi: 10.1002/14651858.CD011029.pub2. Cochrane Database Syst Rev. 2018. PMID: 30306546 Free PMC article.
References
-
- North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) Acute Diarrhea in Children. Flourtown, PA: NASPGHAN; 2007.
-
- World Health Organization. Fact Sheet Number 330. 2013.
-
- Brown KH, Peerson JM, Fontaine O. Use of nonhuman milks in the dietary management of young children with acute diarrhea: a meta-analysis of clinical trials. Pediatrics. 1994;93(1):17–27. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical