Reinterpreting the best biomarker of oxidative stress: The 8-iso-PGF(2α)/PGF(2α) ratio distinguishes chemical from enzymatic lipid peroxidation
- PMID: 25772010
- PMCID: PMC4441846
- DOI: 10.1016/j.freeradbiomed.2015.03.004
Reinterpreting the best biomarker of oxidative stress: The 8-iso-PGF(2α)/PGF(2α) ratio distinguishes chemical from enzymatic lipid peroxidation
Abstract
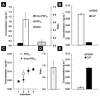
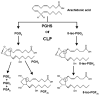
The biomarker 8-iso-prostaglandin F2α (8-iso-PGF2α) is regarded as the gold standard for detection of excessive chemical lipid peroxidation in humans. However, biosynthesis of 8-iso-PGF2α via enzymatic lipid peroxidation by prostaglandin-endoperoxide synthases (PGHSs), which are significantly induced in inflammation, could lead to incorrect biomarker interpretation. To resolve the ambiguity with this biomarker, the ratio of 8-iso-PGF2α to prostaglandin F2α (PGF2α) is established as a quantitative measure to distinguish enzymatic from chemical lipid peroxidation in vitro, in animal models, and in humans. Using this method, we find that chemical lipid peroxidation contributes only 3% to the total 8-iso-PGF2α in the plasma of rats. In contrast, the 8-iso-PGF2α levels in plasma of human males are generated >99% by chemical lipid peroxidation. This establishes the potential for an alternate pathway of biomarker synthesis, and draws into question the source of increases in 8-iso-PGF2α seen in many human diseases. In conclusion, increases in 8-iso-PGF2α do not necessarily reflect increases in oxidative stress; therefore, past studies using 8-iso-PGF2α as a marker of oxidative stress may have been misinterpreted. The 8-iso-PGF2α/PGF2α ratio can be used to distinguish biomarker synthesis pathways and thus confirm the potential change in oxidative stress in the myriad of disease and chemical exposures known to induce 8-iso-PGF2α.
Keywords: Biomarkers; F(2)-isoprostanes; Inflammation; Lipid peroxidation; Oxidative stress.
Published by Elsevier Inc.
Figures

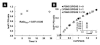
References
-
- Pratico D, Tangirala RK, Rader DJ, Rokach J, FitzGerald GA. Vitamin E suppresses isoprostane generation in vivo and reduces atherosclerosis in ApoE-deficient mice. Nat Med. 1998;4:1189–1192. - PubMed
-
- Keaney JF, Jr, Larson MG, Vasan RS, Wilson PWF, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. - PubMed
-
- Murer SB, Aeberli I, Braegger CP, Gittermann M, Hersberger M, Leonard SW, Taylor AW, Traber MG, Zimmermann MB. Antioxidant supplements reduced oxidative stress and stabilized liver function tests but did not reduce inflammation in a randomized controlled trial in obese children and adolescents. J Nutr. 2014;144:193–201. - PubMed
-
- Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, Fitzgerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ, II, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Van Thiel DH, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett J. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38:698–710. - PubMed
-
- Kadiiska MB, Gladen BC, Baird DD, Graham LB, Parker CE, Ames BN, Basu S, Fitzgerald GA, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ, II, Rokach J, Shigenaga MK, Sun J, Walter PB, Tomer KB, Barrett JC, Mason RP. Biomarkers of oxidative stress study III. Effects of the nonsteroidal anti-inflammatory agents indomethacin and meclofenamic acid on measurements of oxidative products of lipids in CCl4 poisoning. Free Radic Biol Med. 2005;38:711–718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

