Long-term lung transplantation in nonhuman primates
- PMID: 25772308
- PMCID: PMC4564890
- DOI: 10.1111/ajt.13130
Long-term lung transplantation in nonhuman primates
Abstract
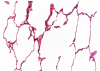
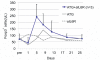
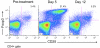
Despite advances in surgical technique and clinical care, lung transplantation still remains a short-term solution for the treatment of end-stage lung disease. To date, there has been limited experience in experimental lung transplantation using nonhuman primate models. Therefore, we have endeavored to develop a long-term, nonhuman primate model of orthotopic lung transplantation for the ultimate purpose of designing protocols to induce tolerance of lung grafts. Here, we report our initial results in developing this model and our observation that the nonhuman primate lung is particularly prone to rejection. This propensity toward rejection may be a consequence of 1) upregulated nonspecific inflammation, and 2) a larger number of pre-existing alloreactive memory T cells, leading to augmented deleterious immune responses. Our data show that triple-drug immunosuppression mimicking clinical practice is not sufficient to prevent acute rejection in nonhuman primate lung transplantation. The addition of horse-derived anti-thymocyte globulin and a monoclonal antibody to the IL-6 receptor allowed six out of six lung recipients to be free of rejection for over 120 days.
Keywords: Immunosuppressant; animal models: nonhuman primate; basic (laboratory) research/science; fusion proteins and monoclonal antibodies; immunosuppressant; lung transplantation/pulmonology; polyclonal preparations.
© Copyright 2015 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures



References
-
- Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth adult lung and heart-lung transplant report–2013; focus theme: Age. J Heart Lung Transplant. 2013;32:965–978. - PubMed
-
- Boskovic S, Kawai T, Smith RN, et al. Monitoring antidonor alloantibodies as a predictive assay for renal allograft tolerance/long-term observations in nonhuman primates. Transplantation. 2006;82:819–825. - PubMed
-
- Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. - PubMed
-
- Lee S, Yamada Y, Boskovic S, Atif M, Tonsho M, Nadazdin O, et al. Renal allograft tolerance can be achieved in non-human primates via delayed mixed-hematopoietic chimerism and alefacept treatment. Abstract , American Transplant Congress, Seattle, 2013, published in Am J Transplant. 2013;13:149.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

