Distinct endotypes of steroid-resistant asthma characterized by IL-17A(high) and IFN-γ(high) immunophenotypes: Potential benefits of calcitriol
- PMID: 25772594
- PMCID: PMC4559139
- DOI: 10.1016/j.jaci.2015.01.026
Distinct endotypes of steroid-resistant asthma characterized by IL-17A(high) and IFN-γ(high) immunophenotypes: Potential benefits of calcitriol
Abstract
Background: A small population of patients with severe asthma does not respond to glucocorticoids (steroid resistant [SR]). They have high morbidity, highlighting an urgent need for strategies to enhance glucocorticoid responsiveness.
Objective: We investigated the immunologic differences between steroid-sensitive (SS) and SR asthmatic patients and the effect on immunophenotype of oral calcitriol treatment because it has been previously shown to beneficially modulate the clinical response to glucocorticoids in patients with SR asthma.
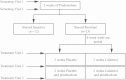
Methods: CD8-depleted PBMCs were isolated from 12 patients with SS and 23 patients with SR asthma and cultured for 7 days with anti-CD3 and IL-2 with or without dexamethasone. Cytokine production was assessed in supernatants by using the Cytometric Bead Array. Patients with SR asthma were subsequently randomized to oral calcitriol or placebo therapy, and identical studies were repeated.
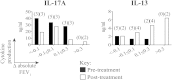
Results: Patients with SR asthma produced significantly increased IL-17A and IFN-γ levels compared with those in patients with SS asthma, although it was evident that cells from individual patients might overproduce one or the other of these cytokines. Production of IL-17A was inversely and production of IL-13 was positively associated with the clinical response to prednisolone. Oral calcitriol, compared with placebo, therapy of the patients with SR asthma significantly improved dexamethasone-induced IL-10 production in vitro while suppressing dexamethasone-induced IL-17A production. This effect mirrored the previously demonstrated improvement in clinical response to oral glucocorticoids in calcitriol-treated patients with SR asthma.
Conclusions: IL-17A(high) and IFN-γ(high) immunophenotypes exist in patients with SR asthma. These data identify immunologic pathways that likely underpin the beneficial clinical effects of calcitriol in patients with SR asthma by directing the SR cytokine profile toward a more SS immune phenotype, suggesting strategies for identifying vitamin D responder immunophenotypes.
Keywords: Asthma; IL-17A; glucocorticoids; steroid resistant; steroid sensitive; vitamin D.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures







References
-
- Wenzel S.E. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. - PubMed
-
- Chung K.F., Wenzel S.E., Brozek J.L., Bush A., Castro M., Sterk P.J. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. - PubMed
-
- Wenzel S., Wilbraham D., Fuller R., Getz E.B., Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007;370:1422–1431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

