Jug r 2-reactive CD4(+) T cells have a dominant immune role in walnut allergy
- PMID: 25772597
- PMCID: PMC4568181
- DOI: 10.1016/j.jaci.2015.01.029
Jug r 2-reactive CD4(+) T cells have a dominant immune role in walnut allergy
Abstract
Background: Allergic reactions to walnut can be life-threatening. Although IgE epitopes of walnut have been studied, CD4(+) T cell-specific epitopes for walnut remain uncharacterized. In particular, the relationship of both phenotype and frequency of walnut-specific T cells to the disease have not been examined.
Objectives: We sought to provide a thorough phenotypic analysis for walnut-reactive T cells in allergic and nonallergic subjects, particularly the relationship of phenotypes and frequencies of walnut-specific T cells with the disease.
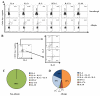
Methods: The CD154 upregulation assay was used to examine CD4(+) T-cell reactivity toward the walnut allergens Jug r 1, Jug r 2, and Jug r 3. A tetramer-guided epitope mapping approach was used to identify HLA-restricted CD4(+) T-cell epitopes in Jug r 2. Direct ex vivo staining with peptide-major histocompatibility complex class II tetramers enabled comparison of the frequency and phenotype of Jug r 2-specific CD4(+) T cells between allergic and nonallergic subjects. Jug r 2-specific T-cell clones were also generated, and mRNA transcription factor levels were assessed by using quantitative RT-PCR. Intracellular cytokine staining assays were performed for further phenotypic analyses.
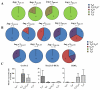
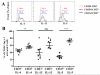
Results: Jug r 2 was identified as the major allergen that elicited CD4(+) T-cell responses. Multiple Jug r 2 T-cell epitopes were identified. The majority of these T cells in allergic subjects have a CCR4(+) phenotype. A subset of these T cells express CCR4(+)CCR6(+) irrespective of the asthmatic status of the allergic subjects. Intracellular cytokine staining confirmed these TH2-, TH2/TH17-, and TH17-like heterogenic profiles. Jug r 2-specific T-cell clones from allergic subjects mainly expressed GATA3, nonetheless, a portion of T-cell clones both GATA3 and RAR-related orphan receptor C (RORC) or RORC alone, confirming the presence of TH2, TH2/TH17, and TH17 cells.
Conclusions: Jug r 2-specific responses dominate walnut T-cell responses in patients with walnut allergy. Jug r 2 central memory CD4(+) cells and terminal effector T cells were detected in peripheral blood, with the central memory phenotype as the most prevalent phenotype. In addition to conventional TH2 cells, TH2/TH17 and TH17 cells were also detected in nonasthmatic and asthmatic patients with walnut allergy. Understanding this T-cell heterogeneity might render better understanding of the disease manifestation.
Keywords: Food allergy; Jug r 2; MHC class II tetramers; T cells; epitopes; walnut.
Copyright © 2015 American Academy of Allergy, Asthma & Immunology. All rights reserved.
Figures


References
-
- Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010 Jun;125(6):1322–6. - PubMed
-
- Fleischer DM, Conover-Walker MK, Matsui EC, Wood RA. The natural history of tree nut allergy. J Allergy Clin Immunol. 2005 Nov;116(5):1087–93. - PubMed
-
- Fleischer DM. The natural history of peanut and tree nut allergy. Curr Allergy Asthma Rep. 2007 Jun;7(3):175–81. - PubMed
-
- Sicherer SH, Furlong TJ, Munoz-Furlong A, Burks AW, Sampson HA. A voluntary registry for peanut and tree nut allergy: characteristics of the first 5149 registrants. J Allergy Clin Immunol. 2001 Jul;108(1):128–32. - PubMed
-
- Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014 Feb;133(2):291–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

