Recombinant virus-like particles elicit protective immunity against avian influenza A(H7N9) virus infection in ferrets
- PMID: 25772674
- PMCID: PMC5805130
- DOI: 10.1016/j.vaccine.2015.03.009
Recombinant virus-like particles elicit protective immunity against avian influenza A(H7N9) virus infection in ferrets
Abstract
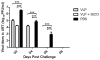
In March 2013, diagnosis of the first reported case of human infection with a novel avian-origin influenza A(H7N9) virus occurred in eastern China. Most human cases have resulted in severe respiratory illness and, in some instances, death. Currently there are no licensed vaccines against H7N9 virus, which continues to cause sporadic human infections. Recombinant virus-like particles (VLPs) have been previously shown to be safe and effective vaccines for influenza. In this study, we evaluated the immunogenicity and protective efficacy of a H7N9 VLP vaccine in the ferret challenge model. Purified recombinant H7N9 VLPs morphologically resembled influenza virions and elicited high-titer serum hemagglutination inhibition (HI) and neutralizing antibodies specific for A/Anhui/1/2013 (H7N9) virus. H7N9 VLP-immunized ferrets subsequently challenged with homologous virus displayed reductions in fever, weight loss, and virus shedding compared to these parameters in unimmunized control ferrets. H7N9 VLP was also effective in protecting against lung and tracheal infection. The addition of either ISCOMATRIX or Matrix-M1 adjuvant improved immunogenicity and protection of the VLP vaccine against H7N9 virus. These results provide support for the development of a safe and effective human VLP vaccine with potent adjuvants against avian influenza H7N9 virus with pandemic potential.
Keywords: Avian influenza; Ferret; Influenza; Vaccine; Virus.
Published by Elsevier Ltd.
Figures



References
-
- Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, et al. Human infection with a novel avian-origin influenza A(H7N9) virus. N Engl J Med. 2013;368:1888–97. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

