Alpha-ketoglutarate dehydrogenase complex-dependent succinylation of proteins in neurons and neuronal cell lines
- PMID: 25772995
- PMCID: PMC4472501
- DOI: 10.1111/jnc.13096
Alpha-ketoglutarate dehydrogenase complex-dependent succinylation of proteins in neurons and neuronal cell lines
Abstract
Reversible post-translation modifications of proteins are common in all cells and appear to regulate many processes. Nevertheless, the enzyme(s) responsible for the alterations and the significance of the modification are largely unknown. Succinylation of proteins occurs and causes large changes in the structure of proteins; however, the source of the succinyl groups, the targets, and the consequences of these modifications on other proteins remain unknown. These studies focused on succinylation of mitochondrial proteins. The results demonstrate that the α-ketoglutarate dehydrogenase complex (KGDHC) can serve as a trans-succinylase that mediates succinylation in an α-ketoglutarate-dependent manner. Inhibition of KGDHC reduced succinylation of both cytosolic and mitochondrial proteins in cultured neurons and in a neuronal cell line. Purified KGDHC can succinylate multiple proteins including other enzymes of the tricarboxylic acid cycle leading to modification of their activity. Inhibition of KGDHC also modifies acetylation by modifying the pyruvate dehydrogenase complex. The much greater effectiveness of KGDHC than succinyl-CoA suggests that the catalysis owing to the E2k succinyltransferase is important. Succinylation appears to be a major signaling system and it can be mediated by KGDHC. Reversible post-translation modifications of proteins are common and may regulate many processes. Succinylation of proteins occurs and causes large changes in the structure of proteins. However, the source of the succinyl groups, the targets, and the consequences of these modifications on other proteins remains unknown. The results demonstrate that the mitochondrial α-ketoglutarate dehydrogenase complex (KGDHC) can succinylate multiple mitochondrial proteins and alter their function. Succinylation appears to be a major signaling system and it can be mediated by KGDHC.
Keywords: Alzheimer's disease; acetylation; brain metabolism; mitochondria; succinylation; α-ketoglutarate dehydrogenase complex.
© 2015 International Society for Neurochemistry.
Conflict of interest statement
The authors have no conflicts of interest.
Figures

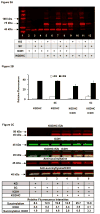
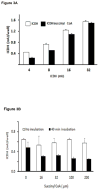




Comment in
-
A new role for α-ketoglutarate dehydrogenase complex: regulating metabolism through post-translational modification of other enzymes.J Neurochem. 2015 Jul;134(1):3-6. doi: 10.1111/jnc.13150. Epub 2015 Jun 4. J Neurochem. 2015. PMID: 26052752 Free PMC article.
References
-
- Brewer GJ, Torricelli JR. Isolation and culture of adult neurons and neurospheres. Nat Protocols. 2007;2:1490–1498. - PubMed
-
- Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: Mechanistic implications. Annals of Neurology. 2005;57:695–703. - PubMed
-
- Buler M, Aatsinki S-M, Izzi V, Uusimaa J, Hakkola J. SIRT5 is under the control of PGC-1α and AMPK and is involved in regulation of mitochondrial energy metabolism. The FASEB Journal 2014 - PubMed
-
- Bunik VI, Denton TT, Xu H, Thompson CM, Cooper AJL, Gibson GE. Phosphonate Analogues of α-Ketoglutarate Inhibit the Activity of the α-Ketoglutarate Dehydrogenase Complex Isolated from Brain and in Cultured Cells†. Biochemistry. 2005;44:10552–10561. - PubMed
-
- Bunik VI, Sievers C. Inactivation of the 2-oxo acid dehydrogenase complexes upon generation of intrinsic radical species. European Journal of Biochemistry. 2002;269:5004–5015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

