Effect of grass pollen immunotherapy on clinical and local immune response to nasal allergen challenge
- PMID: 25773990
- PMCID: PMC4826905
- DOI: 10.1111/all.12608
Effect of grass pollen immunotherapy on clinical and local immune response to nasal allergen challenge
Erratum in
-
Corrigendum.Allergy. 2015 Aug;70(8):1033. doi: 10.1111/all.12659. Allergy. 2015. PMID: 26215719 Free PMC article.
Abstract
Rationale: Nasal allergen provocations may be useful in investigating the pathophysiology of allergic rhinitis and effects of treatments.
Objective: To use grass pollen nasal allergen challenge (NAC) to investigate the effects of allergen immunotherapy in a cross-sectional study.
Methods: We studied nasal and cutaneous responses in untreated subjects with seasonal grass-pollen allergic rhinitis (n = 14) compared with immunotherapy-treated allergics (n = 14), plus a nonatopic control group (n = 14). Volunteers underwent a standardized NAC with 2000 biological units of timothy grass allergen (equivalent to 1.3 μg major allergen, Phl p5). Nasal fluid was collected and analysed by ImmunoCAP and multiplex assays. Clinical response was assessed by symptom scores and peak nasal inspiratory flow (PNIF). Cutaneous response was measured by intradermal allergen injection. Retrospective seasonal symptom questionnaires were also completed.
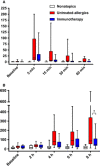
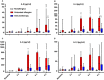
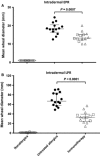
Results: Immunotherapy-treated patients had lower symptom scores (P = 0.04) and higher PNIF (P = 0.02) after challenge than untreated allergics. They had reduced early (P = 0.0007) and late (P < 0.0001) skin responses, and lower retrospective seasonal symptom scores (P < 0.0001). Compared to untreated allergics, immunotherapy-treated patients had reduced nasal fluid concentrations of IL-4, IL-9 and eotaxin (all P < 0.05, 8 h level and/or area under the curve comparison), and trends for reduced IL-13 (P = 0.07, area under the curve) and early-phase tryptase levels (P = 0.06).
Conclusions: Nasal allergen challenge is sensitive in the detection of clinical and biological effects of allergen immunotherapy and may be a useful surrogate marker of treatment efficacy in future studies.
Keywords: Th2; allergic rhinitis; chemokine; cytokine; tryptase.
© 2015 The Authors. Allergy Published by John Wiley & Sons Ltd.
Figures

References
-
- Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J 2004;24:758–764. - PubMed
-
- Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol 2011;106(Suppl):S12–S16. - PubMed
-
- Leynaert B, Neukirch F, Demoly P, Bousquet J. Epidemiologic evidence for asthma and rhinitis comorbidity. J Allergy Clin Immunol 2000;106(Suppl):S201–S205. - PubMed
-
- Scadding GW, Calderon MA, Bellido V, Koed GK, Nielsen NC, Lund K et al. Optimisation of grass pollen nasal allergen challenge for assessment of clinical and immunological outcomes. J Immunol Methods 2012;384:25–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

