Continuous Crystallization of Proteins in a Tubular Plug-Flow Crystallizer
- PMID: 25774098
- PMCID: PMC4353059
- DOI: 10.1021/cg501359h
Continuous Crystallization of Proteins in a Tubular Plug-Flow Crystallizer
Abstract
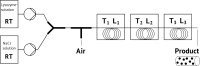
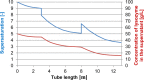
Protein crystals have many important applications in many fields, including pharmaceutics. Being more stable than other formulations, and having a high degree of purity and bioavailability, they are especially promising in the area of drug delivery. In this contribution, the development of a continuously operated tubular crystallizer for the production of protein crystals has been described. Using the model enzyme lysozyme, we successfully generated product particles ranging between 15 and 40 μm in size. At the reactor inlet, a protein solution was mixed with a crystallization agent solution to create high supersaturations required for nucleation. Along the tube, supersaturation was controlled using water baths that divided the crystallizer into a nucleation zone and a growth zone. Low flow rates minimized the effect of shear forces that may impede crystal growth. Simultaneously, a slug flow was implemented to ensure crystal transport through the reactor and to reduce the residence time distribution.
Figures




Similar articles
-
Ultrasound-assisted continuous crystallization of metastable polymorphic pharmaceutical in a slug-flow tubular crystallizer.Ultrason Sonochem. 2023 Nov;100:106627. doi: 10.1016/j.ultsonch.2023.106627. Epub 2023 Sep 30. Ultrason Sonochem. 2023. PMID: 37813044 Free PMC article.
-
Crystal Engineering in Continuous Plug-Flow Crystallizers.Cryst Growth Des. 2017 Dec 6;17(12):6432-6444. doi: 10.1021/acs.cgd.7b01096. Epub 2017 Oct 10. Cryst Growth Des. 2017. PMID: 29234240 Free PMC article.
-
Continuous-flow, well-mixed, microfluidic crystallization device for screening of polymorphs, morphology, and crystallization kinetics at controlled supersaturation.Lab Chip. 2019 Jul 21;19(14):2373-2382. doi: 10.1039/c9lc00343f. Epub 2019 Jun 21. Lab Chip. 2019. PMID: 31222193
-
Do "inhibitors of crystallisation" play any role in the prevention of kidney stones? A critique.Urolithiasis. 2017 Feb;45(1):43-56. doi: 10.1007/s00240-016-0953-y. Epub 2016 Nov 29. Urolithiasis. 2017. PMID: 27900407 Review.
-
Nucleation of solid solutions crystallizing from aqueous solutions.Philos Trans A Math Phys Eng Sci. 2003 Mar 15;361(1804):615-31; discussion 632. doi: 10.1098/rsta.2002.1142. Philos Trans A Math Phys Eng Sci. 2003. PMID: 12662457 Review.
Cited by
-
Mass spectrometric directed system for the continuous-flow synthesis and purification of diphenhydramine.Chem Sci. 2017 Jun 1;8(6):4363-4370. doi: 10.1039/c7sc00905d. Epub 2017 Apr 19. Chem Sci. 2017. PMID: 28979759 Free PMC article.
-
In-drop thermal cycling of microcrystal assembly for senescence control (MASC) with minimal variation in efficacy.Adv Funct Mater. 2023 Sep 12;33(37):2302232. doi: 10.1002/adfm.202302232. Epub 2023 May 1. Adv Funct Mater. 2023. PMID: 37901180 Free PMC article.
-
Antisolvent Crystallization of Telmisartan Using Stainless-Steel Micromixing Membrane Contactors.Cryst Growth Des. 2023 Apr 24;23(5):3720-3730. doi: 10.1021/acs.cgd.3c00123. eCollection 2023 May 3. Cryst Growth Des. 2023. PMID: 37159651 Free PMC article.
-
Production of Cross-Linked Lipase Crystals at a Preparative Scale.Cryst Growth Des. 2021 Mar 3;21(3):1698-1707. doi: 10.1021/acs.cgd.0c01608. Epub 2021 Feb 17. Cryst Growth Des. 2021. PMID: 34602865 Free PMC article.
-
Crystal Shape Modification via Cycles of Growth and Dissolution in a Tubular Crystallizer.Cryst Growth Des. 2018 Aug 1;18(8):4403-4415. doi: 10.1021/acs.cgd.8b00371. Epub 2018 Jun 15. Cryst Growth Des. 2018. PMID: 30918477 Free PMC article.
References
-
- Protein Data Bank in Europe, http://www.rcsb.org (accessed June 9, 2014).
-
- Nanev C. N. Protein crystal nucleation: Recent notions. Cryst. Res. Technol. 2007, 42, 4–12.
-
- Margolin A. L.; Navia M. Proteinkristalle als neue Katalysatoren. Angew. Chem. 2001, 113, 2262–2281.
-
- Jegan Roy J.; Emilia Abraham T. Strategies in Making Cross-Linked Enzyme Crystals. Chem. Rev. 2004, 104, 3705–3722. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
