One-Year Feasibility Study of Replenish MicroPump for Intravitreal Drug Delivery: A Pilot Study
- PMID: 25774328
- PMCID: PMC4356354
- DOI: 10.1167/tvst.3.3.8
One-Year Feasibility Study of Replenish MicroPump for Intravitreal Drug Delivery: A Pilot Study
Abstract
Purpose: To determine the feasibility of the surgical procedure and to collect some safety data regarding the bioelectronics of a novel micro drug pump for intravitreal drug delivery in a Beagle dog model for up to 1 year.
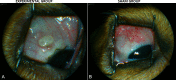
Methods: Thirteen Beagle dogs were assigned to two groups. The experimental group (n = 11) underwent pars plana implantation of MicroPump; the body of which was sutured episclerally, while its catheter was secured at a pars plana sclerotomy. The control group (n = 2) underwent sham surgeries in the form of a temporary suturing of the MicroPump, including placement of the pars plana tube. Baseline and follow-up exams included ophthalmic examination and imaging. The experimental animals were euthanized and explanted at predetermined time points after surgery (1, 3, and 12 months), while the control animals were euthanized at 3 months. All operated eyes were submitted for histopathology.
Results: Eyes were scored according to a modified McDonald-Shadduck system and ophthalmic imaging. Neither the implanted eyes nor the control eyes showed clinically significant pathological changes beyond the expected surgical changes. The operated eyes showed neither significant inflammatory reaction nor tissue ingrowth through the sclerotomy site compared with the fellow eyes.
Conclusion: This study shows that the Replenish Posterior MicroPump could be successfully implanted with good safety profile in this animal model.
Translational relevance: The results of this study in a Beagle dog model are supportive of the biocompatibility of Replenish MicroPump and pave the way to the use of these devices for ocular automated drug delivery after further testing in larger animal models.
Keywords: biocompatibility; intravitreal drug delivery; micropump.
Figures





Similar articles
-
Combined pars plana glaucoma drainage device placement and vitrectomy using a vitrectomy sclerotomy site for tube placement: a case series.BMC Ophthalmol. 2021 Feb 25;21(1):106. doi: 10.1186/s12886-021-01872-z. BMC Ophthalmol. 2021. PMID: 33632169 Free PMC article.
-
Long-term effect on the equine eye of an intravitreal device used for sustained release of cyclosporine A.Vet Ophthalmol. 2000;3(2-3):105-110. doi: 10.1046/j.1463-5224.2000.00117.x. Vet Ophthalmol. 2000. PMID: 11397291
-
A nanoliter resolution implantable micropump for murine inner ear drug delivery.J Control Release. 2019 Mar 28;298:27-37. doi: 10.1016/j.jconrel.2019.01.032. Epub 2019 Jan 25. J Control Release. 2019. PMID: 30690105 Free PMC article.
-
Intravitreal triamcinolone acetonide as an additional tool in pars plana vitrectomy for proliferative diabetic retinopathy.Eur J Ophthalmol. 2003 Jun;13(5):468-73. doi: 10.1177/112067210301300508. Eur J Ophthalmol. 2003. PMID: 12841570 Clinical Trial.
-
Clinical outcomes of pars plana capsulotomy with anterior vitrectomy in pediatric cataract surgery.J AAPOS. 2002 Jun;6(3):163-7. doi: 10.1067/mpa.2002.122148. J AAPOS. 2002. PMID: 12075292
Cited by
-
New Therapeutic Approaches in Diabetic Retinopathy.Rev Diabet Stud. 2015 Spring-Summer;12(1-2):196-210. doi: 10.1900/RDS.2015.12.196. Epub 2015 Aug 10. Rev Diabet Stud. 2015. PMID: 26676668 Free PMC article. Review.
-
Intravitreal infusion: A novel approach for intraocular drug delivery.Sci Rep. 2016 Nov 25;6:37676. doi: 10.1038/srep37676. Sci Rep. 2016. PMID: 27886224 Free PMC article.
-
Extended Duration Vascular Endothelial Growth Factor Inhibition in the Eye: Failures, Successes, and Future Possibilities.Pharmaceutics. 2018 Jan 27;10(1):21. doi: 10.3390/pharmaceutics10010021. Pharmaceutics. 2018. PMID: 29382038 Free PMC article. Review.
-
Biotechnology and Biomaterial-Based Therapeutic Strategies for Age-Related Macular Degeneration. Part I: Biomaterials-Based Drug Delivery Devices.Front Bioeng Biotechnol. 2020 Nov 3;8:549089. doi: 10.3389/fbioe.2020.549089. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33224926 Free PMC article. Review.
-
Intraocular Pressure-Lowering Efficacy of a Sustained-Release Bimatoprost Implant in Dog Eyes Pretreated with Selective Laser Trabeculoplasty.J Ocul Pharmacol Ther. 2022 May;38(4):311-318. doi: 10.1089/jop.2021.0104. Epub 2022 Apr 18. J Ocul Pharmacol Ther. 2022. PMID: 35442770 Free PMC article.
References
-
- Melamud A, Stinnett S, Fekrat S. Treatment of neovascular age-related macular degeneration with intravitreal bevacizumab: efficacy of three consecutive monthly injections. Am J Ophthalmol. 2008;146:91–95. - PubMed
-
- Heier JS, Boyer D, Nguyen QD, et al. The 1-year results of CLEAR-IT 2, a phase 2 study of vascular endothelial growth factor trap-eye dosed as-needed after 12-week fixed dosing. Ophthalmology. 2011;118:1098–1106. - PubMed
-
- Lally DR, Gerstenblith AT, Regillo CD. Preferred therapies for neovascular age-related macular degeneration. Curr Opin Ophthalmol. 2012;23:182–188. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

