Severe myopathy in mice lacking the MEF2/SRF-dependent gene leiomodin-3
- PMID: 25774500
- PMCID: PMC4396495
- DOI: 10.1172/JCI80115
Severe myopathy in mice lacking the MEF2/SRF-dependent gene leiomodin-3
Abstract
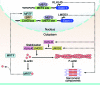
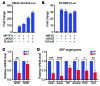
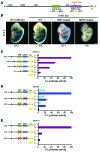
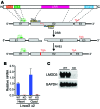
Maintenance of skeletal muscle structure and function requires a precise stoichiometry of sarcomeric proteins for proper assembly of the contractile apparatus. Absence of components of the sarcomeric thin filaments causes nemaline myopathy, a lethal congenital muscle disorder associated with aberrant myofiber structure and contractility. Previously, we reported that deficiency of the kelch-like family member 40 (KLHL40) in mice results in nemaline myopathy and destabilization of leiomodin-3 (LMOD3). LMOD3 belongs to a family of tropomodulin-related proteins that promote actin nucleation. Here, we show that deficiency of LMOD3 in mice causes nemaline myopathy. In skeletal muscle, transcription of Lmod3 was controlled by the transcription factors SRF and MEF2. Myocardin-related transcription factors (MRTFs), which function as SRF coactivators, serve as sensors of actin polymerization and are sequestered in the cytoplasm by actin monomers. Conversely, conditions that favor actin polymerization de-repress MRTFs and activate SRF-dependent genes. We demonstrated that the actin nucleator LMOD3, together with its stabilizing partner KLHL40, enhances MRTF-SRF activity. In turn, SRF cooperated with MEF2 to sustain the expression of LMOD3 and other components of the contractile apparatus, thereby establishing a regulatory circuit to maintain skeletal muscle function. These findings provide insight into the molecular basis of the sarcomere assembly and muscle dysfunction associated with nemaline myopathy.
Figures


References
-
- North KN, Ryan MM. Nemaline Myopathy. GeneReviews. Seattle, Washington, USA: University of Washington; 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL077439/HL/NHLBI NIH HHS/United States
- R01 DK099653/DK/NIDDK NIH HHS/United States
- DK-099653/DK/NIDDK NIH HHS/United States
- T32 GM008014/GM/NIGMS NIH HHS/United States
- U01 HL100401/HL/NHLBI NIH HHS/United States
- T32 GM007062/GM/NIGMS NIH HHS/United States
- R01 HL111665/HL/NHLBI NIH HHS/United States
- HL-077439/HL/NHLBI NIH HHS/United States
- U01-HL-100401/HL/NHLBI NIH HHS/United States
- HL-111665/HL/NHLBI NIH HHS/United States
- HL-093039/HL/NHLBI NIH HHS/United States
- 5T32GM007062-40/GM/NIGMS NIH HHS/United States
- R01 HL093039/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

