A methodological framework for drug development in rare diseases
- PMID: 25774598
- PMCID: PMC4255937
- DOI: 10.1186/s13023-014-0164-y
A methodological framework for drug development in rare diseases
Abstract
Introduction: Developing orphan drugs is challenging because of their severity and the requisite for effective drugs. The small number of patients does not allow conducting adequately powered randomized controlled trials (RCTs). There is a need to develop high quality, ethically investigated, and appropriately authorized medicines, without subjecting patients to unnecessary trials.
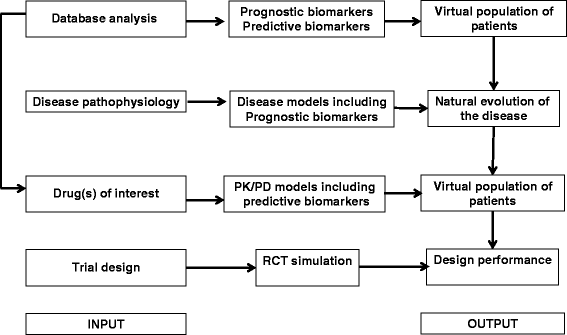
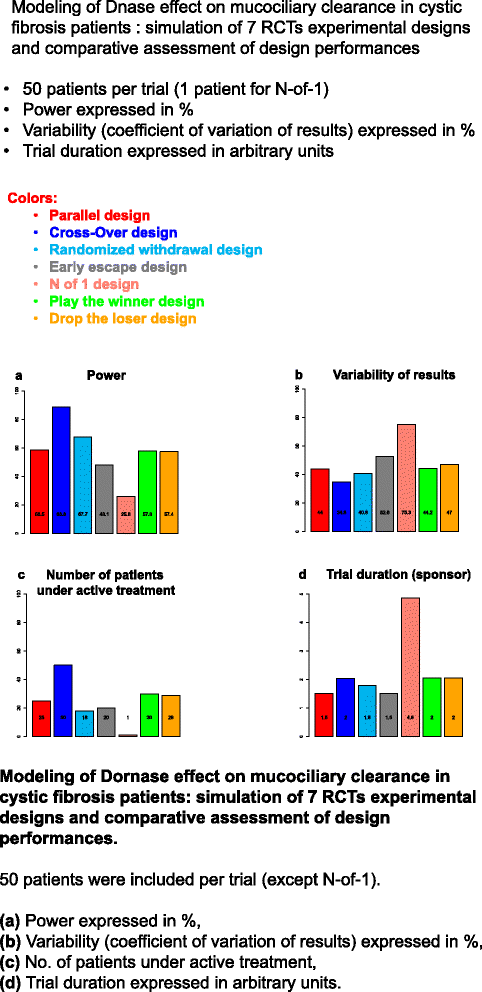
Aims and objectives: The main aim is to develop generalizable framework for choosing the best-performing drug/endpoint/design combinations in orphan drug development using an in silico modeling and trial simulation approach. The two main objectives were (i) to provide a global strategy for each disease to identify the most relevant drugs to be evaluated in specific patients during phase III RCTs, (ii) and select the best design for each drug to be used in future RCTs.
Methodological approach: In silico phase III RCT simulation will be used to find the optimal trial design and was carried out in two steps: (i) statistical analysis of available clinical databases and (ii) integrative modeling that combines mathematical models for diseases with pharmacokinetic-pharmacodynamics models for the selected drug candidates.
Conclusion: There is a need to speed up the process of orphan drug development, develop new methods for translational research and personalized medicine, and contribute to European Medicines Agency guidelines. The approach presented here offers many perspectives in clinical trial conception.
Figures
References
-
- EURORDIS. What is a rare disease? (http://www.eurordis.org/content/what-rare-disease) Accessed on: 31 October. 2011.
-
- EURORDIS:Rare diseases: understanding this public health priority european organisation for rare diseases (http://www.eurordis.org/IMG/pdf/princeps_document-EN.pdf) Accessed on: 17 February. 2013.
-
- Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials. St Louis: Mosby-Year Book; 1996.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

