Force transmission during adhesion-independent migration
- PMID: 25774834
- PMCID: PMC6485532
- DOI: 10.1038/ncb3134
Force transmission during adhesion-independent migration
Abstract
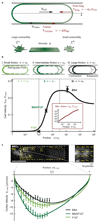
When cells move using integrin-based focal adhesions, they pull in the direction of motion with large, ∼100 Pa, stresses that contract the substrate. Integrin-mediated adhesions, however, are not required for in vivo confined migration. During focal adhesion-free migration, the transmission of propelling forces, and their magnitude and orientation, are not understood. Here, we combine theory and experiments to investigate the forces involved in adhesion-free migration. Using a non-adherent blebbing cell line as a model, we show that actin cortex flows drive cell movement through nonspecific substrate friction. Strikingly, the forces propelling the cell forward are several orders of magnitude lower than during focal-adhesion-based motility. Moreover, the force distribution in adhesion-free migration is inverted: it acts to expand, rather than contract, the substrate in the direction of motion. This fundamentally different mode of force transmission may have implications for cell-cell and cell-substrate interactions during migration in vivo.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

